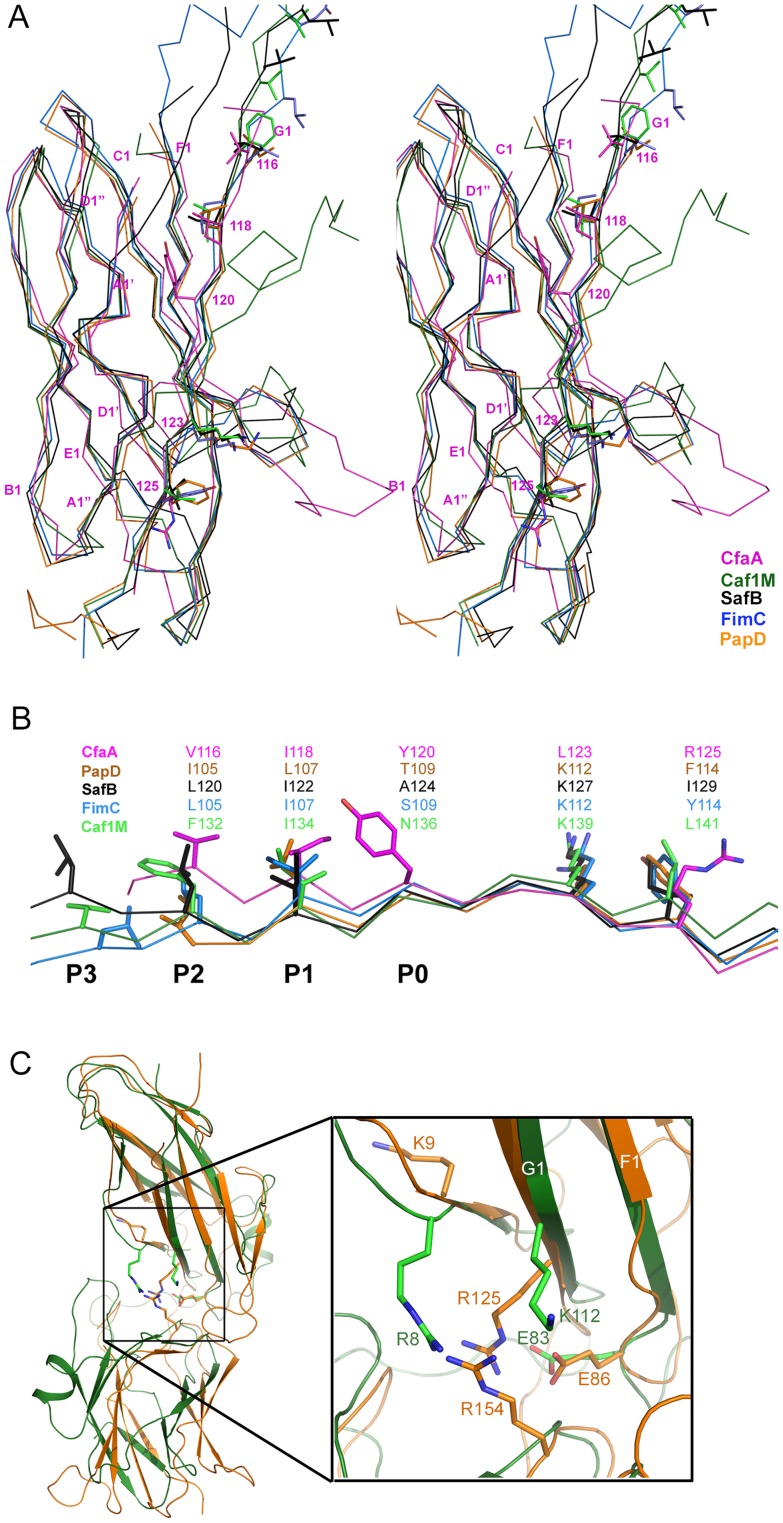
Figure 2. Structure comparison of chaperones from different families.
(A) Stereoscopic pair showing the superposition of structures of chaperone N-terminal domains. Five structures are included: CfaA (magenta), Caf1M (green), SafB (black), FimC (blue) and PapD (coral). All β-strands are labeled. Subunit interacting residues on the G1 strand are shown as stick models and numbered. (B) Detailed alignment of the donor strand for CfaA, Caf1M, SafB, FimC and PapD, showing alternating hydrophobic residues at positions P0 (hydrophobic, CfaA only) and P1 to P3. (C) Structure comparison of CfaA and PapD. Structures of CfaA (coral) and PapD (green) are superimposed and the cleft region between the two domains is magnified. Charged residues K9, E86, R125, and R154 from CfaA are shown as stick models with carbon atoms color in coral. Those from PapD R8, E83, and K112 are similarly illustrated with carbon in green. Oxygen atoms are in red and nitrogen in blue.