Abstract
AIM: To compare a possible relation between Helicobacter pylori (H. pylori) and the oxygen- and nitrogen radical system in humans.
METHODS: Mechanisms for H. pylori to interfere with the oxygen and nitrogen radical system is of great importance for understanding of the H. pylori persistence and pathogenesis. Biopsies were obtained from the gastric wall of 21 individuals. Ongoing infection with H. pylori was detected using direct analyze from the biopsies using campylobacter-like organism test (CLO-test) and/or by using 14C-urea breath test. The individuals were divided in a negative H. pylori and a positive H. pylori group. Expression in the gastric mucosa of inducible nitric oxide syntase (iNOS), nicotinamide adenine dinucleotide phosphate-oxidase (NADPH-oxidase) myeloperoxidase (MPO), and nitrotyrosine were assessed by Western blotting.
RESULTS: The individuals who undervent gastroscopy were divided in a H. pylori neg. [n = 13, m/f = 7/6, age (mean) = 39] and a H. pylori pos. group [n= 8, m/f = 5/3, age (mean) = 53]. Using western blot analysis iNOS was detected as a 130 kDa band. The iNOS expression was upregulated in the antrum of H. pylori infected individuals in comparison to the controls, mean ± SD being 12.6 ± 2.4 vs 8.3 ± 3.1, P < 0.01. There was a markedly upregulated expression of MPO in the antrum of H. pylori infected individuals in comparison to the control group without infection. In several of non-infected controls it was not possible to detect any MPO expression at all, whereas the expression was high in all the infected subjects, mean ± SD being 5.1 ± 3.4 vs 2.1 ± 1.9, P < 0.05. The NADPH-oxidase expression was analysed by detecting the NADPH-oxidase subunit p47-phox expression. P47-phox was detected as a 47 kDa band using Western blot, and showed a significantly higher expression of p47-phox in the antrum of the H. pylori infected individuals compared to the controls, mean ± SD being 3.1 ± 2.2 vs 0.3 ± 0.2, P < 0.01. Regarding nitrotyrosine formation, Western blot did not show any significant increase or decrease compared to controls, 7.0 ± 0.9 vs 6.9 ± 1.1, not significant.
CONCLUSION: iNOS, MPO and NADPH-oxidase was up-regulated among H. pylori infected. Regarding nitrotyrosine no difference was found. This support an H. pylori related inhibition of radical formation.
Keywords: Helicobacter pylori, Radical, Myeloperoxidase, Nicotinamide adenine dinucleotide phosphate, Nitrotyrosine, Gastric
Core tip: The present project was performed to compare a possible relation between Helicobacter pylori (H. pylori) and the oxygen- and nitrogen radical system in humans. Expression of inducible nitric oxide syntase, myeloperoxidase and nicotinamide adenine dinucleotide phosphate-oxidase was upregulted in the antrum of the group with H. pylori infection. Regarding nitrotyrosine formation, Western blot did not show any significant increase or decrease compared to controls. The present study illustrates the complex picture of the oxidative stress in relation to H. pylori infection. The present study supports the theory of an H. pylori related inhibition of the enzymes involved in the oxy- and/or nitro- radical formation pathway.
INTRODUCTION
Helicobacter pylori (H. pylori) is a pathogen colonizing the human gastric mucosa playing a significant role in the development of gastric ulcer, gastritis, and gastric cancer[1] Until recently there was insufficient knowledge about how H. pylori could avoid being eliminated by the acute host defence and establish a chronic infection in the gastric mucosa of humans. Recent studies have shown that H. pylori interferes with reactive oxygen species (ROS) such as superoxide anion (O2-) that is of importance in the elimination of invading microorganisms[2,3]. At the same time reactive nitrogen intermediates such as nitric oxide (NO) represent another class of oxidants. NO can be formed as a nitrogenous product of nitric oxide synthase (NOS). Peroxynitrite, formed by NO and O2-, is a very powerful oxidant. It is unstable with dimensions related to the hydroxyl radical[4]. In neutrophils and macrophages large amounts of reactive oxygen and nitrogen species are presented to the invading microorganism. Neutrophils phagocytose bacteria into the intracellular phagosome, where an eruption of reactive species results in bacterial destruction. During successful conditions the bacteria is eliminated and there is no extracellular oxidant generation[5].
However H. pylori persist in the gastric mucosa, causing a chronic infection that increases the risk for pathological changes such as adenocarcinoma. Therefore the mechanisms for H. pylori to interfere with the oxygen and nitrogen radical system is of great importance for understanding the persistence and pathogenesis of H. pylori.
We and others have pointed out the association between H. pylori infection and an increased mucosal expression of iNOS both in humans and in mongolian gerbils[6-8]. Despite what one could expect, the juxtamucosal level of nitric oxide (NO) is lower in the infected than in the uninfected stomach[7,8]. We have shown that there is an inhibition of nitrotyrosine expression, being a reflection of the formation of peroxynitrite, in H. pylori infected Mongolian gerbils despite upregulated formation of both myeloperoxidase (MPO) and inducible nitric oxide synthase (iNOS)[6]. Results from in vivo registration of NO and hydrogen peroxide (H2O2) on Mongolian gerbils substantiates the fact infection with H. pylori reduces levels of NO[9]. It is recently suggested that specific proteins contained by H. pylori enables the pathogen to cope with the damaging effects of NO. These systems are suggested to be a part in the microbial protection against nitrosative stress[10]. Several traditional anti-inflammatory drugs have been shown to have an effect on epithelial cells infected by H. pylori by inhibiting the induction of iNOS by suppressing the activation of NADPH oxidase[11] .
The present project was performed to further compare a possible relation between H. pylori and the oxygen- and nitrogen radical system in humans.
Special interest was on the suspected upregulation on the enzymatic oxy- and nitro radical systems, and if this would result in an increased radical formation. To evaluate activity of peroxynitrite, expression of nitrotyrosine was used as an indicator of radical formation.
MATERIALS AND METHODS
Ethics approval
Approval was obtained from the Research Ethics Committee at Sahlgrenska Academy, Gothenburg University and from the Gothenburg Regional Ethical Review Board.
Study groups
Gastric biopsies were obtained from the antral wall of 21 individuals. The individuals were divided in a H. pylori neg. [n = 13, m/f = 7/6, age (mean) = 39] and a H. pylori pos. group [n = 8, m/f = 5/3, age (mean) = 53]. Gastro-esophageal reflux (GER) was diagnosed in one subject in the H. pylori pos group and in four subjects in the H. pylori neg group. Ulcer in the duodenum was found in two individuals in the H. pylori pos group.
Diagnostic procedures
Ongoing infection with H. pylori was detected using direct analyze from the biopsies using campylobacter-like organism test (CLO-test) and/or by using 14C-urea breath test[12].
Western blot
Biopsies were collected during gastroscopy. The samples were snap-frozen in nitrogenum liquidum and kept for further analysis at -70 °C. Sonication (Sonifier 450/250, Branson Ultrasonics Co. Danbury, United States) or homogenization (Polytron, PT-MR 2100, Kinematica) was performed of all samples at 2 °C in a PE-buffer (10 mmol/L potassium Phosphate buffer, pH 6.8, and 1 mmol/L EDTA) containing CHAPS {3-[(3-cholamidopropyl) dimethyl-ammanio] 1-propanesulfonate}, aprotinin (1 μg/mL), leupeptin (10 μg/ mL), pepstatin (10 μg/mL) and Pefablock (1 mg/mL) (Boeringer Mannheim, Mannheim, Germany). All samples were centrifugated at 10.000 g for 10 min at 4 °C. Analysation was performed of the supernatant for protein content using the method of Bradford12 and then kept at -70 °C for future analysis. Samples were diluted in SDS-buffer and heated at 70 °C for 10 min before they were loaded on a NuPage 10% BisTris gel (Invitrogen, Carlsbad, CA, United States). One lane of each gel was loaded with prestained molecular weight standards (SeeBlue™, Invitrogen, Carlsbad, CA, United States). Following the electrophoresis the proteins were transferred to a polyvinyldifluoride membrane (Amersham, Buckinghamshire, United Kingdom) which was incubated with antibodies directed against iNOS, MPO, NADPH-oxidase or nitrotyrosine containing proteins. For identifying iNOS the NOS2 (H-174) sc-8310 (Santa Cruz Biotechnology inc) antibody was used. It is a rabbit polyclonal antibody raised against a recombinant protein corresponding to amino acids 2-175 mapping at the amino terminus of iNOS of human origin. Lack of cross-reaction with nNOS or eNOS was reported by manufacturer. Antibody Anti-myeloperoxidase 07-496 lot 24587 (Upstate, Lake Placid, NY, United States) was used for detecting MPO. This is a rabbit antibody that recognizes MPO subunits at 12 and 60 kDa. In the present study the 60 kDa band was used for quantification of the protein. Anti-nitrotyrosine rabbit immunoaffinity purified IgG catalog 06-284, lot 26427 (Upstate, Lake Placid, NY, United States) was used to assess nitrated proteins. For identifying NADPH-oxidase the p47-phox (H-195) sc 14015 (Santa Cruz Biotechnology inc.) was used. This is a rabbit polyclonal antibody raised against amino acids 196-390 of p47-phox of human origin. P47-phox is required for activation of NADPH-oxidase in neutrophils and other phagocytic cells. During activation of NADPH-oxidase, p47-phox migrate to the plasma membrane where it associates with other subunits to form the active complex. Goat anti-rabbit antibodies were used to identify immunoreactive proteins by chemiluminescence [iNOS, NADPH-oxidase (p47-phox) and nitrotyrosine; goat-anti rabbit sc 2007(Santa Cruz, CA, United States)] [MPO; IgG 12-448 (Upstate Lake Placid, NY, United States)]. CDP-Star (Tropix, Bedford, MA, United States) was used as a substrate. Images were captured by a LAS-100 cooled CCD-camera (Fujifilm, Tokyo, Japan) and semi-quantification was performed using the soft ware Gauge 3.3 (Fujifilm, Tokyo, Japan). As positive controls, to confirm the identity of the protein, RAW 264.7 (sc 2212, Santa Cruz Biotech) was used for iNOS, HL60 was used for MPO and NADPH-oxidase (p47-phox).For nitrotyrosine the immunoblotting control (12-354, Upstate) was used.
Statistical analysis
Statistical analysis was performed using non parametric Mann-Whitney U-test. P-values of < 0.05 were regarded as being of statistical significance.
RESULTS
Inducible nitric oxide synthase
Using western blot analysis iNOS was detected as a 130 kDa band. The iNOS expression was upregulated in the antrum of H. pylori infected individuals in comparison to the control group without infection as shown in Figure 1A, mean ± SD being 12.6 ± 2.4 vs 8.3 ± 3.1, P < 0.01. Western blot detecting iNOS with a band at 130 kDa in RAW 264.7 (pos contr.), and in human antral mucosa retrieved from H. pylori pos. and H. pylori neg. volunteers during endoscopy is shown in Figure 2.
Figure 1.
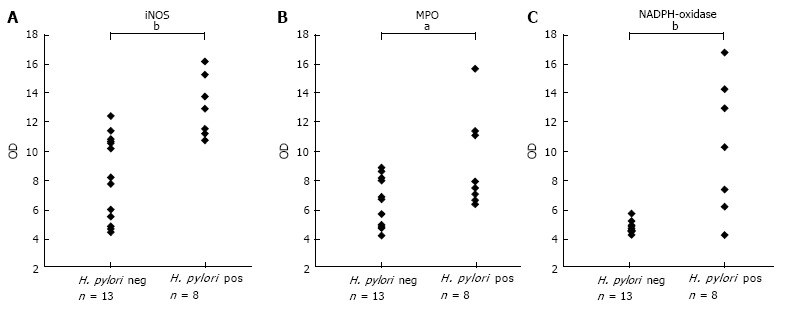
Scatter-plot demonstrating the result of Western blot inducible nitric oxide synthase, myeloperoxidase and nicotinamide adenine dinucleotide phosphate-oxidase protein expression in biopsies from the anrum of the Helicobacter pylori neg (n = 13) and Helicobacter pylori pos (n = 8) groups. A: Inducible nitric oxide synthase (iNOS), bP < 0.01; B: Myeloperoxidase (MPO), aP < 0.05; C: Nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase subunit p47-phox in biopsies from the antrum of the H. pylori neg. (n = 13) and H. pylori pos. (n = 8) groups, bP < 0.01, OD: Optical density.
Figure 2.
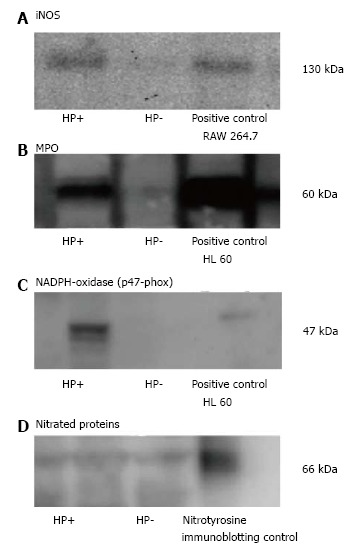
Western blot. A: Western blot detecting inducible nitric oxide syntase (iNOS) with a band at 130 kDa in RAW 264.7 (pos contr.), and in human antral mucosa retrieved from Helicobacter pylori (H. pylori) pos. and H. pylori neg. volunteers during endoscopy; B: Western blot of the MPO positive 60 kDa band in the positive HL60 control and in gastric mucosal specimens of H. pylori pos. and H. pylori neg. volunteers; C: Western blot of p47-phox, representing NADPH-oxidase with a band at 47 kDa (pos. contr.) HL60, and in HP+ and HP- samples; D: Regarding Western blot representing Nitrotyrosine, several bands of Nitrated proteins could be analysed. Shown in the figure is a typical 66 kDa band in the pos. control and in H. pylori pos. and H. pylori neg. subjects.
Myeloperoxidase
As shown in Figure 1B, MPO expression was markedly upregulated in the antrum of the H. pylori infected individuals in comparison to the control group without infection, mean ± SD being 5.1 ± 3.4 vs 2.1 ± 1.9, P < 0.05. In several of the non-infected controls it was not possible to detect any MPO expression at all, whereas the expression was high in all the infected subjects. Western blot of the MPO positive 60 kDa band in the positive HL60 control and in gastric mucosal specimens of H. pylori pos. and H. pylori neg. volunteers is shown in Figure 2.
NADPH-oxidase
The expression of NADPH-oxidase was analysed by detecting the NADPH-oxidase subunit p47-phox expression. P47-phox was detected as a 47 kDa band using Western blot. Figure 1C shows a significantly higher expression of p47-phox in the the antrum of H. pylori infected individuals in comparison to the control group without infection, mean ± SD being 3.1 ± 2.2 vs 0.3 ± 0.2, P < 0.01. P47-phox was low in all non-infected controls. In the H. pylori infected subjects there was a large spreading of the p47-phox expression. A typical Western blot result is shown in Figure 2.
Nitrotyrosine
Western blot analysis did not show any significant increase nor decrease in nitrotyrosine expression the antrum of H. pylori infected individuals in comparison to the control group without infection, 7.0 ± 0.9 vs 6.9 ± 1.1, not significant (Figure 3). Regarding Western blot representing Nitrotyrosine, several bands of Nitrated proteins could be analysed. Shown in the Figure 2 is a typical 66 kDa band in the positive control and in H. pylori pos. and H. pylori neg. subjects.
Figure 3.
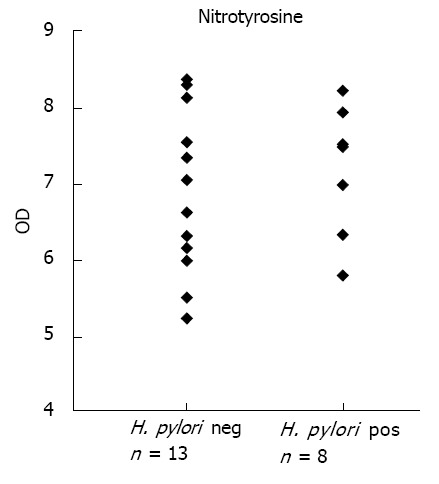
Nitrotyrosine. Scatter-plot demonstrating the result of Western blot analysis of nitrotyrosine formation in biopsies from the antrum of the Helicobacter pylori (H. pylori) neg. (n = 13) and H. pylori pos. (n = 8) groups. There were no significant changes between groups. OD: Optical density.
DISCUSSION
The findings of the present investigation can confirm that H. pylori infection in humans is related to an up regulation of the expression of MPO, iNOS and NADPH-oxidase in the human gastric mucosa. Furthermore the study shows that there are no significant changes in levels of proteins containing nitrotyrosine compared to non-infected subjects following this up-regulation. This finding confirms the results from our studies on Mongolian gerbils, and supports the theory of an H. pylori related inhibition of radical formation[6,9].
Studying the early stages of H. pylori infecting the stomach is important for understanding the evolution of pathology such as carcinogenesis. Using an animal model makes it possible to assess different stages of pathological development in an experimental setting. However it is important to evaluate the experimental findings in a human population before making any conclusions regarding H. pylori infection in human gastric mucosa.
The initial host reaction to the H. pylori infection is the same as to any bacterial infection: Phagocytic neutrophils and monocytes are recruited to the infected tissue and consume oxygen that is converted to O2- by NADPH-oxidase, and then dismutated to H2O2[13]. Activation of neutrophils results in the release of MPO, which catalyzes the oxidation of electron donors by H2O2[14]. A complex is formed that is responsible for the production of powerful oxidants with potential to react with a large variety of substances[15,16]. For example, MPO-H2O2 reacts with chloride to form hypochlorus acid and subsequently the oxidative chloramines are formed. The MPO-H2O2-chloride system is responsible for many biological effects, both beneficial and negative for the host[17].
In general, inflammation results in invading epithelial cells and macrophages leading to a marked expression of iNOS and resulting in generation of NO[17].
Several studies have described an increase in iNOS production following H. pylori infection in both humans and animal models[6-8,18-20]. Some have suggested that the up-regulated iNOS production following H. pylori infection would lead to an increase in NO production which could result in the increase of DNA damage and apoptosis[18-21]. It has been suggested that classification of iNOS expression in the gastric mucosa could be used clinically to identify patients with a high risk for gastric cancer[22]. The host will try to terminate the infection by activating the mucosal generation of the oxy- and nitro-radical forming enzymes the resulting in formation of the cytotoxic peroxynitrite. In the extracellular space NO released from macrophages can eliminate H. pylori[23]. An effective increase of production of NO and oxy-radicals would lead to eradication of the bacteria. However H. pylori persist in the host, causing a chronic inflammatory reaction that instead in the long run may be deleterious to the host. The fact that H. pylori survives in this hostile environment despite up regulation of iNOS suggests that the pathogen has developed strategies to avoid NO-dependent eradication. An increasing number of studies have reported about the complexity of the H. pylori response to oxidative and nitrogen stress[24,25].
H. pylori may also have a direct effect on reduction in gastric mucosal blood flow by inhibiting NO production by iNOS and thereby reducing the vasodilatory and mast cell stabilizing effect of NO[26].
We have by use of electrochemical microelectrodes in vivo confirmed reduction of intraluminal NO in Mongolian gerbils following infection with H. pylori[9]. Reduced levels of NO could be explained by inhibition of iNOS activity[27]. Helicobacter produced arginase has been proposed as one of the ways for H. pylori to inhibit NO production[23,25]. H. pylori may also produce asymmetrical dimethyl arginine (ADMA ) that can block iNOS by competitive inhibition. ADMA is a methylated form of arginine that has been found to be significantly up-regulated in the human antrum of H. pylori positive individuals[8,28]. Another explanation for reduced NO levels could be scavenge of NO by reacting with reactive oxygen species (ROS)[7,18]. A result of this reaction would be an increase in the production of peroxynitrite, and resulting in increased levels of nitrotyrosine. Thus nitrotyrosine can be used to indicate peroxinitrite activity over time. The present investigation as well as previous studies on H. pylori infection in Mongolian gerbil demonstrates a significant up regulation of the formation of iNOS and MPO, but no significant changes in the levels of nitrotyrosine[6]. These findings strongly support the theory supports the theory of an H. pylori related inhibition of radical formation at an enzymatic level of NO generation.
The present study does not provide data on if H. pylori also inhibit the oxy-radical forming enzymes. Oxidative stress could potentially have a negative effect on the capacity of H. pylori to infest the human stomach. However it is shown that H. pylori produces a number of antioxidative proteins, the most described ones being bacterial produced superoxide dismutase (SOD)[29]. SOD production is described as being of importance for H. pylori being able to grow and survive in a situation with oxidative stress, and is regarded as a factor being of importance for of the microbial colonization of the stomach. Catalase and arginase are other examples of antioxidant proteins produced by H. pylori that might contribute to bacterial survival under conditions of oxidative stress[23,30,31].
Taken together the present study illustrates the complex picture of the oxidative stress response to H. pylori infection. The nitro- and oxy-radical formation systems are up-regulated following infection and inflammation. This up-regulation is to be regarded as an attempt from the host to eradicate the bacteria. However, long standing up-regulation of the reactive oxygen- and nitrogen species will also lead to tissue damage and a risk of carcinogenesis. This study supports the theory supports the theory of an H. pylori related inhibition the. The mechanisms behind how the bacteria and the impotent host defence act to induce DNA- and tissue damaging effects need to be further explored.
The results suggest that there is a relationship between inhibition of formation of ROS and reactive nitrogen species and H. pylori being able to survive in the human gastric mucosa.
COMMENTS
Background
Helicobacter pylori (H. pylori) colonization of the mucosal space of the stomach causes a chronic infection resulting in the development of pathological changes such as adenocarcinoma. Mechanisms for H. pylori to interfere with the oxygen and nitrogen radical system is of great importance for understanding the persistence and pathogenesis of H. pylori.
Research frontiers
Several studies have described an increase in inducible nitric oxide syntase (iNOS) production following H. pylori infection in both humans and animal models. An effective increase of production of NO and oxy-radicals would lead to eradication of the bacteria. The fact that H. pylori survives in this hostile environment despite up regulation of iNOS suggests that the pathogen has developed strategies to avoid NO-dependent eradication. The findings of the present investigation can confirm that H. pylori infection in humans is related to an up regulation of the expression of MPO, iNOS and NADPH-oxidase in the human gastric mucosa. Furthermore the study shows that there are no significant changes in levels of proteins containing nitrotyrosine compared to non-infected subjects following this up-regulation.
Breakthroughs and innovations
The investigation presented here illustrates the complex picture of the oxidative stress response to H. pylori infection. The nitro- and oxy-radical formation systems are up-regulated following infection and inflammation. This up-regulation is to be regarded as an attempt from the host to eradicate the bacteria. However, long standing up-regulation of the reactive oxygen- and nitrogen species will also lead to tissue damage and a risk of carcinogenesis. This study supports the theory of an H. pylori related inhibition of formation of reactive oxygen species (ROS) and reactive nitrogen species. The mechanisms behind how the bacteria and the impotent host defence act to induce DNA- and tissue damaging effects need to be further explored.
Applications
By understanding how H. pylori manages not to be extinguished in the hostile environment by hindering the formation of reactive oxygen species and reactive nitrogen intermediates we will gain a greater understanding of the mechanisms involved in H. pylori related disease.
Terminology
Myeloperoxidase (MPO) is an enzyme of importance in the microbicidal role of phagocytes. iNOS was first identified in macrophages. iNOS is involved in the production of NO, but has also many other functions. Nicotinamide adenine dinucleotide phosphate-oxidase (NADPH-oxidase) is a transmembrane electron transport chain involved in the production of different ROS. Nitrotyrosine can be used as marking the activity of peroxynitrite.
Peer review
In this study the authors demonstrated, in human gastric mucosa of H. pylori positive patients, an increase of some enzymes belonging to oxidative stress pathway, while the amount of nitrotyrosine rich proteins did not differ from H. pylori negative tissues.
Footnotes
Supported by The grants financially from the Swedish Medical Research Council and the Gothenburg Medical Society
P- Reviewer: Balaban YH, Cardaropoli S, Zhu YL S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
References
- 1.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 2.Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 3.Kawahara T, Kohjima M, Kuwano Y, Mino H, Teshima-Kondo S, Takeya R, Tsunawaki S, Wada A, Sumimoto H, Rokutan K. Helicobacter pylori lipopolysaccharide activates Rac1 and transcription of NADPH oxidase Nox1 and its organizer NOXO1 in guinea pig gastric mucosal cells. Am J Physiol Cell Physiol. 2005;288:C450–C457. doi: 10.1152/ajpcell.00319.2004. [DOI] [PubMed] [Google Scholar]
- 4.Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci USA. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas MJ, Hedrick CC, Smith S, Pang J, Jerome WG, Willard AS, Shirley PS. Superoxide generation by the human polymorphonuclear leukocyte in response to latex beads. J Leukoc Biol. 1992;51:591–596. doi: 10.1002/jlb.51.6.591. [DOI] [PubMed] [Google Scholar]
- 6.Elfvin A, Bölin I, Lönroth H, Fändriks L. Gastric expression of inducible nitric oxide synthase and myeloperoxidase in relation to nitrotyrosine in Helicobacter pylori-infected Mongolian gerbils. Scand J Gastroenterol. 2006;41:1013–1018. doi: 10.1080/00365520600633537. [DOI] [PubMed] [Google Scholar]
- 7.Iguchi M, Shiotani A, Nishioka S. Helicobacter pylori infection reduces intraluminal nitric oxide. Scand J Gastroenterol. 2000;35:694–698. doi: 10.1080/003655200750023345. [DOI] [PubMed] [Google Scholar]
- 8.von Bothmer C, Edebo A, Lönroth H, Olbe L, Pettersson A, Fändriks L. Helicobacter pylori infection inhibits antral mucosal nitric oxide production in humans. Scand J Gastroenterol. 2002;37:404–408. doi: 10.1080/003655202317316024. [DOI] [PubMed] [Google Scholar]
- 9.Elfvin A, Edebo A, Bölin I, Fändriks L. Quantitative measurement of nitric oxide and hydrogen peroxide in Helicobacter pylori-infected Mongolian gerbils in vivo. Scand J Gastroenterol. 2007;42:1175–1181. doi: 10.1080/00365520701288306. [DOI] [PubMed] [Google Scholar]
- 10.Justino MC, Ecobichon C, Fernandes AF, Boneca IG, Saraiva LM. Helicobacter pylori has an unprecedented nitric oxide detoxifying system. Antioxid Redox Signal. 2012;17:1190200. doi: 10.1089/ars.2011.4304. [DOI] [PubMed] [Google Scholar]
- 11.Cho SO, Lim JW, Kim H Red ginseng extract inhibits the expression of MCP-1 and iNOS in Helicobacter pylori-infected gastric epithelial cells by suppressing the activation of NADPH oxidase and Jak2/Stat3. J Ethnopharmacol. 2013;150:761–764. doi: 10.1016/j.jep.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Hamlet AK, Erlandsson KI, Olbe L, Svennerholm AM, Backman VE, Pettersson AB. A simple, rapid, and highly reliable capsule-based 14C urea breath test for diagnosis of Helicobacter pylori infection. Scand J Gastroenterol. 1995;30:1058–1063. doi: 10.3109/00365529509101607. [DOI] [PubMed] [Google Scholar]
- 13.Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–3017. [PubMed] [Google Scholar]
- 14.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- 15.Hataishi R, Kobayashi H, Takahashi Y, Hirano S, Zapol WM, Jones RC. Myeloperoxidase-associated tyrosine nitration after intratracheal administration of lipopolysaccharide in rats. Anesthesiology. 2002;97:887–895. doi: 10.1097/00000542-200210000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 17.Jaiswal M, LaRusso NF, Gores GJ. Nitric oxide in gastrointestinal epithelial cell carcinogenesis: linking inflammation to oncogenesis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G626–G634. doi: 10.1152/ajpgi.2001.281.3.G626. [DOI] [PubMed] [Google Scholar]
- 18.Goto T, Haruma K, Kitadai Y, Ito M, Yoshihara M, Sumii K, Hayakawa N, Kajiyama G. Enhanced expression of inducible nitric oxide synthase and nitrotyrosine in gastric mucosa of gastric cancer patients. Clin Cancer Res. 1999;5:1411–1415. [PubMed] [Google Scholar]
- 19.Mannick EE, Bravo LE, Zarama G, Realpe JL, Zhang XJ, Ruiz B, Fontham ET, Mera R, Miller MJ, Correa P. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 1996;56:3238–3243. [PubMed] [Google Scholar]
- 20.Son HJ, Rhee JC, Park DI, Kim YH, Rhee PL, Koh KC, Paik SW, Choi KW, Kim JJ. Inducible nitric oxide synthase expression in gastroduodenal diseases infected with Helicobacter pylori. Helicobacter. 2001;6:37–43. doi: 10.1046/j.1523-5378.2001.00004.x. [DOI] [PubMed] [Google Scholar]
- 21.Li CQ, Pignatelli B, Ohshima H. Increased oxidative and nitrative stress in human stomach associated with cagA+ Helicobacter pylori infection and inflammation. Dig Dis Sci. 2001;46:836–844. doi: 10.1023/a:1010764720524. [DOI] [PubMed] [Google Scholar]
- 22.Naito Y, Takagi T, Okada H, Nukigi Y, Uchiyama K, Kuroda M, Handa O, Kokura S, Yagi N, Kato Y, et al. Expression of inducible nitric oxide synthase and nitric oxide-modified proteins in Helicobacter pylori-associated atrophic gastric mucosa. J Gastroenterol Hepatol. 2008;23 Suppl 2:S250–S257. doi: 10.1111/j.1440-1746.2008.05412.x. [DOI] [PubMed] [Google Scholar]
- 23.Gobert AP, McGee DJ, Akhtar M, Mendz GL, Newton JC, Cheng Y, Mobley HL, Wilson KT. Helicobacter pylori arginase inhibits nitric oxide production by eukaryotic cells: a strategy for bacterial survival. Proc Natl Acad Sci USA. 2001;98:13844–13849. doi: 10.1073/pnas.241443798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nardone G, Rocco A, Malfertheiner P. Review article: helicobacter pylori and molecular events in precancerous gastric lesions. Aliment Pharmacol Ther. 2004;20:261–270. doi: 10.1111/j.1365-2036.2004.02075.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang G, Alamuri P, Maier RJ. The diverse antioxidant systems of Helicobacter pylori. Mol Microbiol. 2006;61:847–860. doi: 10.1111/j.1365-2958.2006.05302.x. [DOI] [PubMed] [Google Scholar]
- 26.Henriksnäs J, Atuma C, Phillipson M, Sandler S, Engstrand L, Holm L. Acute effects of Helicobacter pylori extracts on gastric mucosal blood flow in the mouse. World J Gastroenterol. 2009;15:219–225. doi: 10.3748/wjg.15.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Bothmer C, Bölin I, Pettersson A, Fändriks L. Stimulated murine macrophages as a bioassay for H. pylori-related inhibition of nitric oxide production. Scand J Gastroenterol. 2003;38:380–386. doi: 10.1080/00365520310000816. [DOI] [PubMed] [Google Scholar]
- 28.Fändriks L, von Bothmer C, Johansson B, Holm M, Bölin I, Pettersson A. Water extract of Helicobacter pylori inhibits duodenal mucosal alkaline secretion in anesthetized rats. Gastroenterology. 1997;113:1570–1575. doi: 10.1053/gast.1997.v113.pm9352859. [DOI] [PubMed] [Google Scholar]
- 29.Seyler RW, Olson JW, Maier RJ. Superoxide dismutase-deficient mutants of Helicobacter pylori are hypersensitive to oxidative stress and defective in host colonization. Infect Immun. 2001;69:4034–4040. doi: 10.1128/IAI.69.6.4034-4040.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris AG, Hazell SL: Localisation of Helicobacter pylori catalase in both the periplasm and cytoplasm, and its dependence on the twin-arginine target protein, KapA, for activity. FEMS microbiology letters. 2003;229:283–289. doi: 10.1016/S0378-1097(03)00850-4. [DOI] [PubMed] [Google Scholar]
- 31.McGee DJ, Radcliff FJ, Mendz GL, Ferrero RL, Mobley HL. Helicobacter pylori rocF is required for arginase activity and acid protection in vitro but is not essential for colonization of mice or for urease activity. J Bacteriol. 1999;181:7314–7322. doi: 10.1128/jb.181.23.7314-7322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


