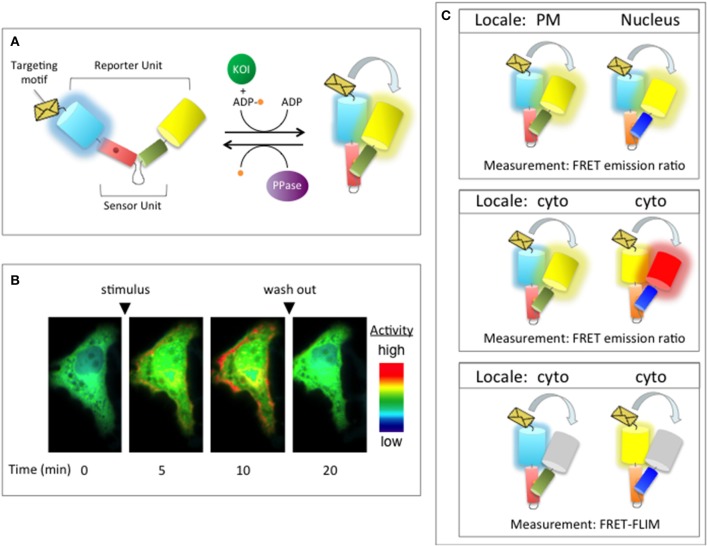
Figure 4.
Genetically-targetable FRET-based biosensors. (A) Design of a FRET-based kinase activity reporter based on an engineered molecular switch. In this design, the sensor unit is composed of a PAABD (red cylinder) tethered to a substrate domain (green cylinder) that is specifically phosphorylated by the kinase-of-interest (KOI; green circle). The sensor unit is sandwiched between the reporter unit, which is comprised of two FPs that are able to undergo FRET [e.g., CFP (cyan cylinder) and YFP (yellow cylinder)]. A targeting motif (orange envelope) is used to direct the reporter to distinct subcellular regions. In the unphosphorylated state, the FPs are far removed from one another and, therefore, do not undergo FRET. An increase in the activity of the KOI leads to a phosphorylation-dependent conformational change that alters the distance and/or orientation of the FPs, increasing FRET between them. Phosphatase (PPase; purple oval)-mediated dephosphorylation of the reporter switches it back to the open conformation, reducing FRET. (B) Pseudocolor images of a live cell imaging experiment. In this experiment, a time-dependent increase in the FRET emission ratio (YFP FRET/CFP) is observed following stimulation with a pharmacological activator (stimulus). Upon removal of the stimulus (wash out), the emission ratio returns to basal levels. Warmer colors represent high activity while cooler colors indicate low activity. (C) Several ways in which genetically-targetable biosensors can be used to monitor real-time changes in the activity profiles of two or more signaling enzymes in the same cell. Top panel: Activity reporters that utilize the same FP FRET pair can be monitored simultaneously in the same cell provided that each biosensor is targeted to a distinct subcellular locale, such as the plasma membrane (PM) and the nucleus. Middle and bottom panels: To track the activities of two or more signaling enzymes in the same subcellular region, activity reporters that utilize spectrally distinct FP FRET pairs (e.g., CFP/YFP and YFP/RFP) can be used (middle panel) or alternative fluorescence imaging techniques, such as FRET-fluorescence lifetime imaging (FRET-FLIM), which only measures changes in emission of the donor fluorophore, can be employed (bottom panel). Cyto, cytoplasm; PM, plasma membrane.