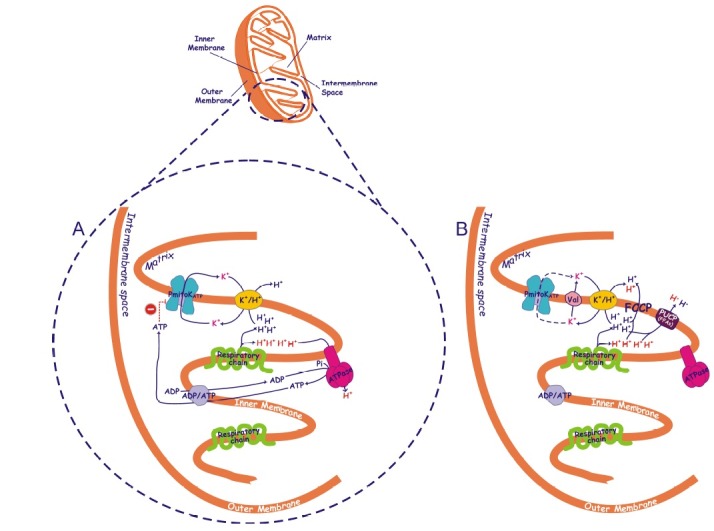
Fig. 1. Possible mechanism of coupling in the absence of measurable protonmotive force mediated by the plant mitochondrial potassium channel. A simplified picture of a durum wheat mitochondrion is reported, with mitochondrial cristae enlarged to schematize the presence in the inner mitochondrial membrane of respiratory chain, ATP synthase (ATPase), ADP/ATP and K+/H+ antiporters and ATP-inhibited plant mitochondrial potassium channel (PmitoKATP) (A) as well as of K+ ionophore valinomycin (Val), plant uncoupling protein (PUCP) activated by free fatty acids (FFAs) and chemical uncoupler carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone (FCCP) (B). Partial inhibition of PmitoKATP by ATP occurring at the cytosolic face of the channel (1), may control the extent of channel activity. In the presence of high K+ concentration, the potassium cycle deriving from the cooperation of the PmitoKATP (A) or valinomycin (B) with K+/H+ antiporter may partly or fully uncouple mitochondria, respectively. Protons ejected by the respiratory chain in the course of substrate oxidation are reported in blue and in red; the first ones contribute to the measurable bulk phase ΔΨ and ΔpH, while the second ones are assumed to represent a latent non-classically measurable, localized, protonmotive force. The controlled cooperation of PmitoKATP with K+/H+ antiporter may collapse measurable bulk phase ΔΨ and ΔpH without excluding the ATP synthase pathway (A). When uncontrolled K+ uptake by valinomycin bypasses ATP brake, classical uncoupling is observed involving all protons and excluding ATP synthase (B). As expected, uncoupling is also observed when FFAs or FCCP are used (B). The scheme does not consider topology of proteins and interaction sites. For detailed explanation see the text.