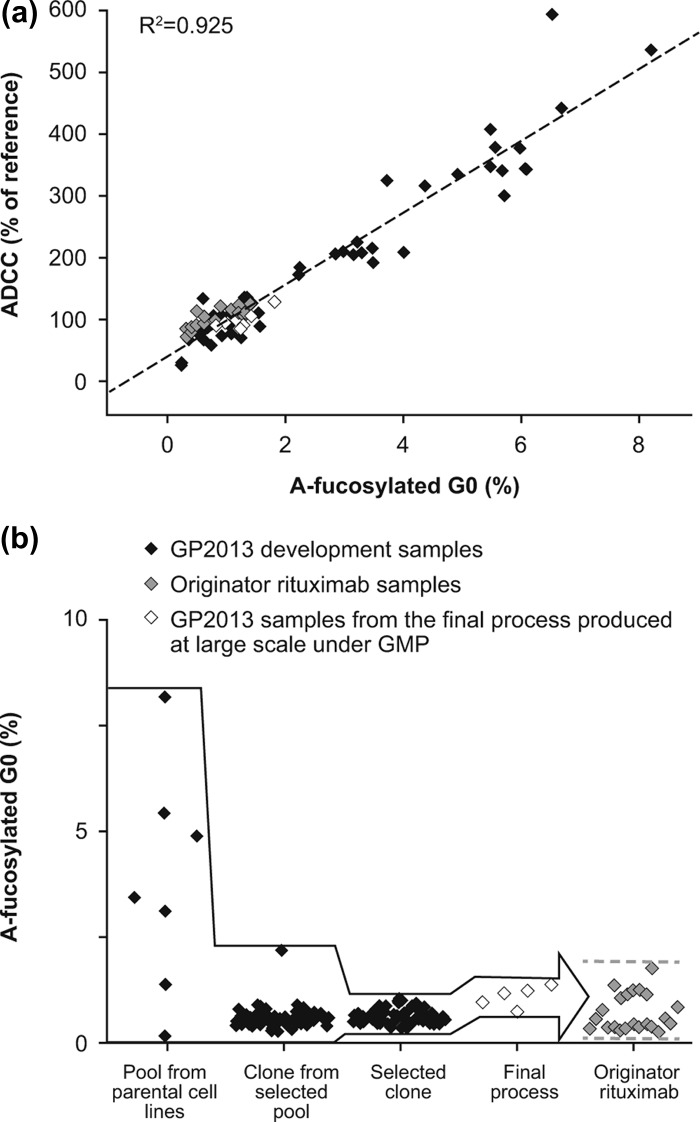
Figure 1.
(a) The wide range of a-fucosylated bG0 glycans in GP2013 development samples allowed the establishment of a quantitative structure–function relationship between a-fucosylated bG0 and ADCC. Black squares: GP2013 development samples; gray squares: rituximab originator samples; white squares: GP2013 samples from the final process produced on a large scale under good manufacturing practice (GMP). (b) Target-directed development of GP2013 to ensure that a-fucosylated bG0, and thus ADCC, is within the originator target range. The different steps entailed: (1) selection and subsequent cloning of a pool from a parental Chinese hamster ovary (CHO) cell line with a good overall quality and productivity profile and an a-fucosylated bG0 value closest to originator rituximab; (2) selection of the clone with the best overall quality profile, with a-fucosylated bG0 structure being close to the originator and showing little variation; (3) exposing the selected clone to different process conditions to optimize the overall quality and productivity profile resulting in the selection of a final process; (4) GMP production of GP2013 on a large scale using the final process, resulting in a-fucosylated bG0 values in the middle of the originator range.