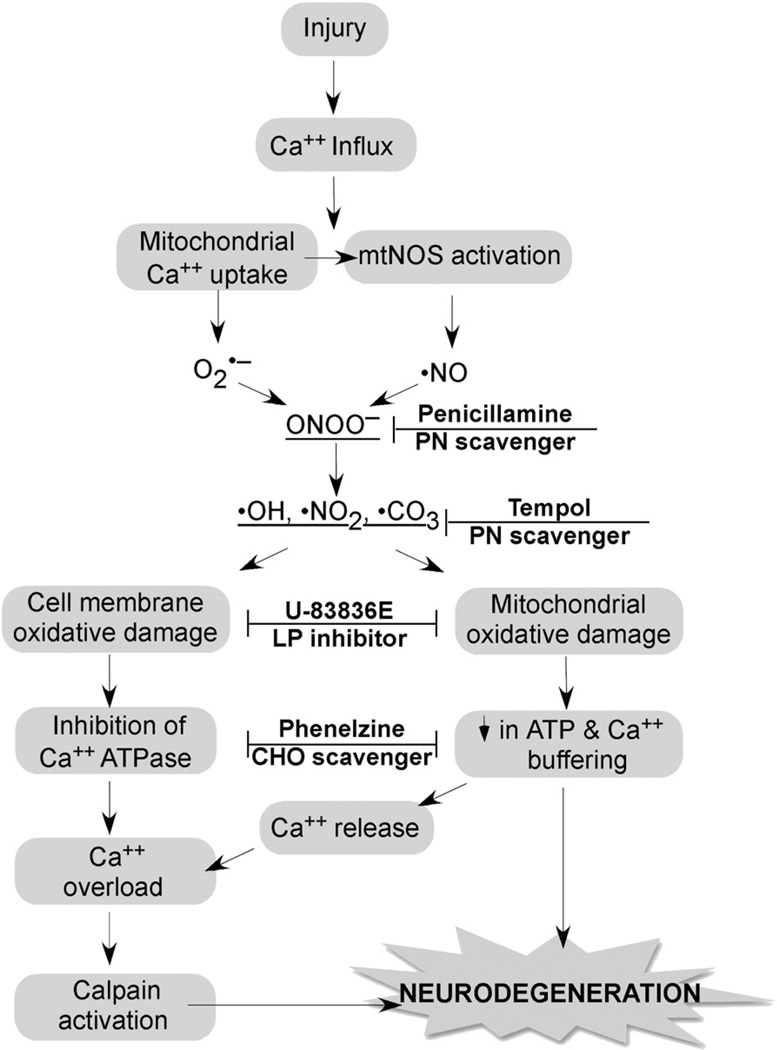
Fig. 2.
Strategy for combination antioxidant therapy for TBI and SCI. Injury triggers an increase in cytoplasmic Ca2+ via voltage dependent and glutamate receptor-operated channels, which initiates the depicted cascade of events. Mitochondria Ca2+ uptake causes O2•− leakage from the electron transport chain and activation of mitochondrial nitric oxide synthase (NOS). O2•− and •NO combine to form the highly reactive nitrogen species PN (ONOO−) giving rise to nitrogen dioxide •NO2, hydroxyl •OH and •CO3. These PN-derived radicals induce cell membrane and mitochondrial oxidative damage resulting in the inhibition of Ca2+ ATPase and a decrease in the mitochondrial ATP production and membrane potential (ΔΨ), respectively. Mitochondrial dysfunction causes the dumping of mitochondrial Ca2+ into the cytoplasm where it exacerbates cytoplasmic calcium overload and calpain activation. Calpain initiates the proteolysis of cytoskeletal proteins and other substrates ultimately contributing to neurodegeneration. The combination of the antioxidant penicillamine or tempol, which catalytically reacts with PN-derived radicals with a chain-breaking LP inhibitor such as U-83836E or a carbonyl (CHO) scavenging compound (Phenelzine) should produce a better neuroprotective effect than any of these compounds alone.