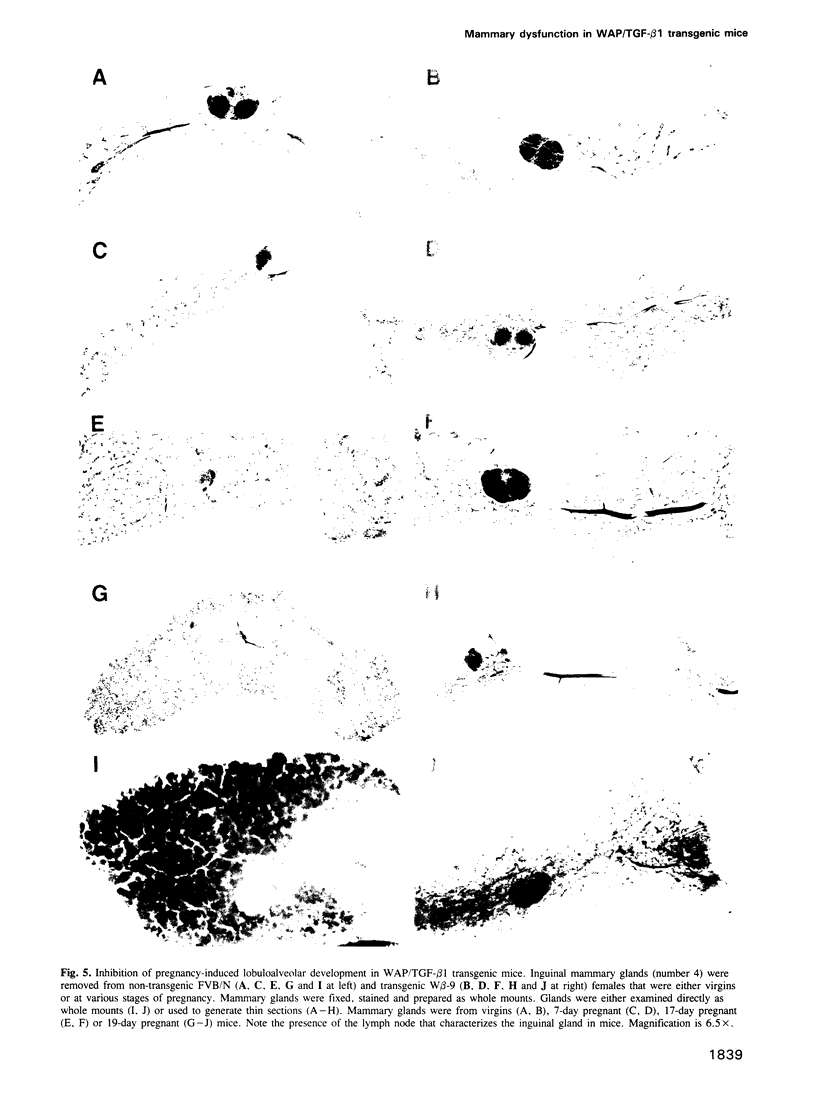
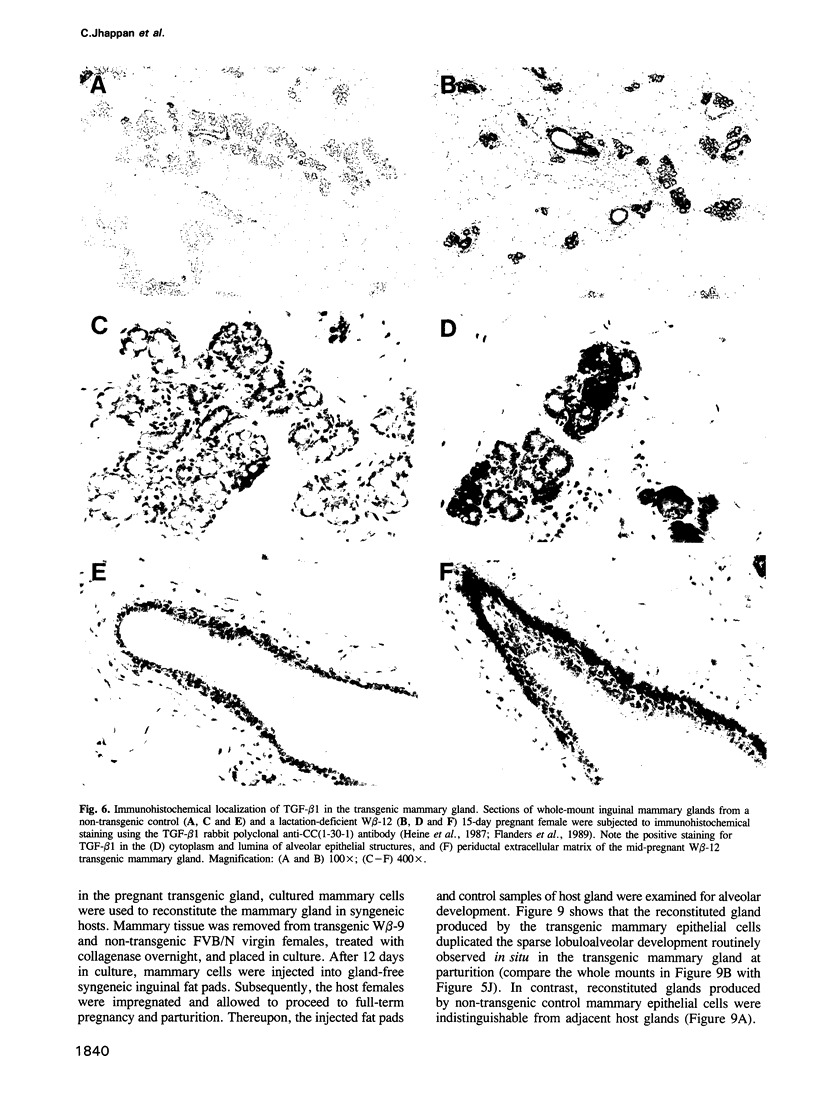
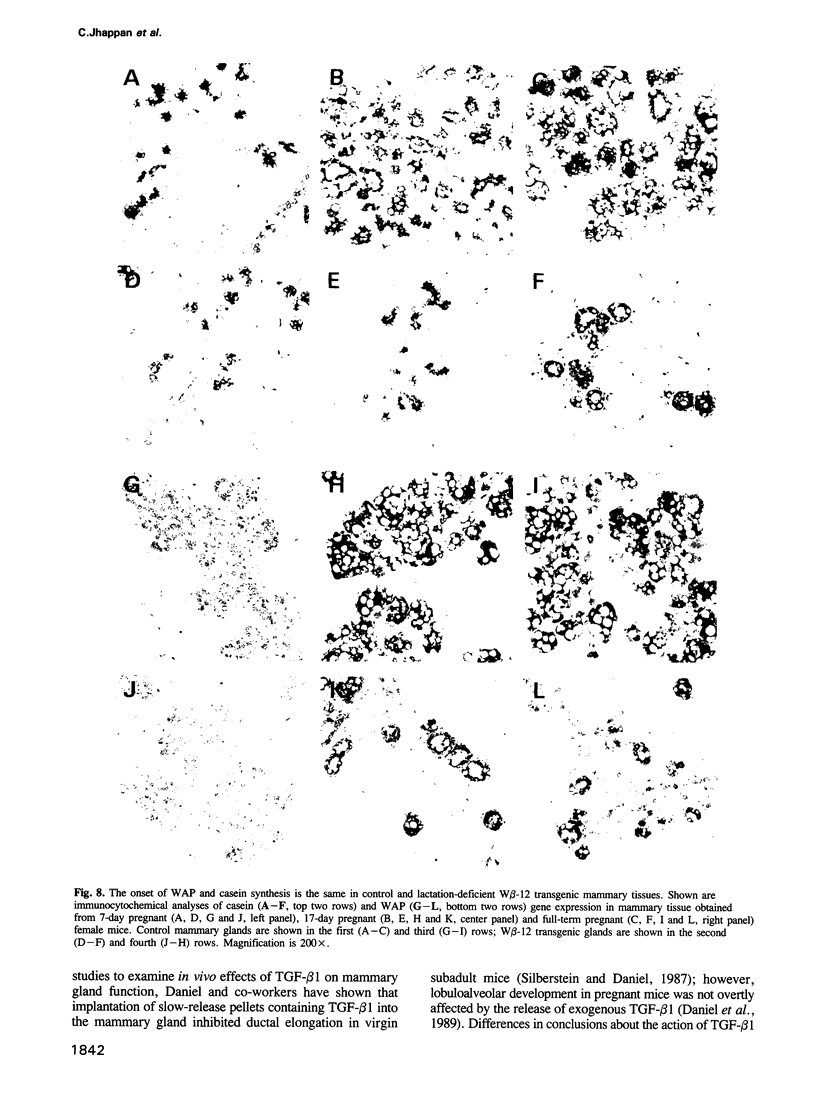
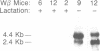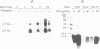Abstract
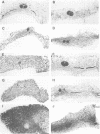
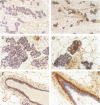
Transforming growth factor-beta 1 (TGF-beta 1) possesses highly potent, diverse and often opposing cell-specific activities, and has been implicated in the regulation of a variety of physiologic and developmental processes. To determine the effects of in vivo overexpression of TGF-beta 1 on mammary gland function, transgenic mice were generated harboring a fusion gene consisting of the porcine TGF-beta 1 cDNA placed under the control of regulatory elements of the pregnancy-responsive mouse whey-acidic protein (WAP) gene. Females from two of four transgenic lines were unable to lactate due to inhibition of the formation of lobuloalveolar structures and suppression of production of endogenous milk protein. In contrast, ductal development of the mammary glands was not overtly impaired. There was a complete concordance in transgenic mice between manifestation of the lactation-deficient phenotype and expression of RNA from the WAP/TGF-beta 1 transgene, which was present at low levels in the virgin gland, but was greatly induced at mid-pregnancy. TGF-beta 1 was localized to numerous alveoli and to the periductal extracellular matrix in the mammary gland of transgenic females late in pregnancy by immunohistochemical analysis. Glands reconstituted from cultured transgenic mammary epithelial cells duplicated the inhibition of lobuloalveolar development observed in situ in the mammary glands of pregnant transgenic mice. Results from this transgenic model strongly support the hypothesis that TGF-beta 1 plays an important in vivo role in regulating the development and function of the mammary gland.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Sporn M. B. Type beta transforming growth factor in human platelets: release during platelet degranulation and action on vascular smooth muscle cells. J Cell Biol. 1986 Apr;102(4):1217–1223. doi: 10.1083/jcb.102.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard J. A., Lyons R. M., Moses H. L. The cell biology of transforming growth factor beta. Biochim Biophys Acta. 1990 Jun 1;1032(1):79–87. doi: 10.1016/0304-419x(90)90013-q. [DOI] [PubMed] [Google Scholar]
- Brunner A. M., Marquardt H., Malacko A. R., Lioubin M. N., Purchio A. F. Site-directed mutagenesis of cysteine residues in the pro region of the transforming growth factor beta 1 precursor. Expression and characterization of mutant proteins. J Biol Chem. 1989 Aug 15;264(23):13660–13664. [PubMed] [Google Scholar]
- Burdon T., Wall R. J., Shamay A., Smith G. H., Hennighausen L. Over-expression of an endogenous milk protein gene in transgenic mice is associated with impaired mammary alveolar development and a milchlos phenotype. Mech Dev. 1991 Dec;36(1-2):67–74. doi: 10.1016/0925-4773(91)90073-f. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Daniel C. W., Silberstein G. B., Van Horn K., Strickland P., Robinson S. TGF-beta 1-induced inhibition of mouse mammary ductal growth: developmental specificity and characterization. Dev Biol. 1989 Sep;135(1):20–30. doi: 10.1016/0012-1606(89)90154-1. [DOI] [PubMed] [Google Scholar]
- Danielpour D., Kim K. Y., Dart L. L., Watanabe S., Roberts A. B., Sporn M. B. Sandwich enzyme-linked immunosorbent assays (SELISAs) quantitate and distinguish two forms of transforming growth factor-beta (TGF-beta 1 and TGF-beta 2) in complex biological fluids. Growth Factors. 1989;2(1):61–71. doi: 10.3109/08977198909069082. [DOI] [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Goeddel D. V. The murine transforming growth factor-beta precursor. J Biol Chem. 1986 Apr 5;261(10):4377–4379. [PubMed] [Google Scholar]
- Flanders K. C., Thompson N. L., Cissel D. S., Van Obberghen-Schilling E., Baker C. C., Kass M. E., Ellingsworth L. R., Roberts A. B., Sporn M. B. Transforming growth factor-beta 1: histochemical localization with antibodies to different epitopes. J Cell Biol. 1989 Feb;108(2):653–660. doi: 10.1083/jcb.108.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaumenhaft R., Abe M., Mignatti P., Rifkin D. B. Basic fibroblast growth factor-induced activation of latent transforming growth factor beta in endothelial cells: regulation of plasminogen activator activity. J Cell Biol. 1992 Aug;118(4):901–909. doi: 10.1083/jcb.118.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth I. A. The mammary gland. Baillieres Clin Endocrinol Metab. 1991 Dec;5(4):809–832. doi: 10.1016/s0950-351x(10)80016-3. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Transgenic mice as probes into complex systems. Science. 1989 Dec 8;246(4935):1265–1275. doi: 10.1126/science.2686032. [DOI] [PubMed] [Google Scholar]
- Heine U., Munoz E. F., Flanders K. C., Ellingsworth L. R., Lam H. Y., Thompson N. L., Roberts A. B., Sporn M. B. Role of transforming growth factor-beta in the development of the mouse embryo. J Cell Biol. 1987 Dec;105(6 Pt 2):2861–2876. doi: 10.1083/jcb.105.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. J., Strain A. J., Elstow S. F., Swenne I., Milner R. D. Bi-functional action of transforming growth factor-beta on DNA synthesis in early passage human fetal fibroblasts. J Cell Physiol. 1986 Aug;128(2):322–328. doi: 10.1002/jcp.1041280226. [DOI] [PubMed] [Google Scholar]
- Jhappan C., Stahle C., Harkins R. N., Fausto N., Smith G. H., Merlino G. T. TGF alpha overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell. 1990 Jun 15;61(6):1137–1146. doi: 10.1016/0092-8674(90)90076-q. [DOI] [PubMed] [Google Scholar]
- Keski-Oja J., Blasi F., Leof E. B., Moses H. L. Regulation of the synthesis and activity of urokinase plasminogen activator in A549 human lung carcinoma cells by transforming growth factor-beta. J Cell Biol. 1988 Feb;106(2):451–459. doi: 10.1083/jcb.106.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondaiah P., Van Obberghen-Schilling E., Ludwig R. L., Dhar R., Sporn M. B., Roberts A. B. cDNA cloning of porcine transforming growth factor-beta 1 mRNAs. Evidence for alternate splicing and polyadenylation. J Biol Chem. 1988 Dec 5;263(34):18313–18317. [PubMed] [Google Scholar]
- Kulkarni A. B., Huh C. G., Becker D., Geiser A., Lyght M., Flanders K. C., Roberts A. B., Sporn M. B., Ward J. M., Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence D. A., Pircher R., Krycève-Martinerie C., Jullien P. Normal embryo fibroblasts release transforming growth factors in a latent form. J Cell Physiol. 1984 Oct;121(1):184–188. doi: 10.1002/jcp.1041210123. [DOI] [PubMed] [Google Scholar]
- Lehnert S. A., Akhurst R. J. Embryonic expression pattern of TGF beta type-1 RNA suggests both paracrine and autocrine mechanisms of action. Development. 1988 Oct;104(2):263–273. doi: 10.1242/dev.104.2.263. [DOI] [PubMed] [Google Scholar]
- Lin H. Y., Wang X. F., Ng-Eaton E., Weinberg R. A., Lodish H. F. Expression cloning of the TGF-beta type II receptor, a functional transmembrane serine/threonine kinase. Cell. 1992 Feb 21;68(4):775–785. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- Massagué J. Receptors for the TGF-beta family. Cell. 1992 Jun 26;69(7):1067–1070. doi: 10.1016/0092-8674(92)90627-o. [DOI] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Merlino G. T., Stahle C., Jhappan C., Linton R., Mahon K. A., Willingham M. C. Inactivation of a sperm motility gene by insertion of an epidermal growth factor receptor transgene whose product is overexpressed and compartmentalized during spermatogenesis. Genes Dev. 1991 Aug;5(8):1395–1406. doi: 10.1101/gad.5.8.1395. [DOI] [PubMed] [Google Scholar]
- Merlino G. T. Transgenic animals in biomedical research. FASEB J. 1991 Nov;5(14):2996–3001. doi: 10.1096/fasebj.5.14.1752364. [DOI] [PubMed] [Google Scholar]
- Mieth M., Boehmer F. D., Ball R., Groner B., Grosse R. Transforming growth factor-beta inhibits lactogenic hormone induction of beta-casein expression in HC11 mouse mammary epithelial cells. Growth Factors. 1990;4(1):9–15. doi: 10.3109/08977199009011005. [DOI] [PubMed] [Google Scholar]
- Moses H. L., Yang E. Y., Pietenpol J. A. TGF-beta stimulation and inhibition of cell proliferation: new mechanistic insights. Cell. 1990 Oct 19;63(2):245–247. doi: 10.1016/0092-8674(90)90155-8. [DOI] [PubMed] [Google Scholar]
- Oberhammer F. A., Pavelka M., Sharma S., Tiefenbacher R., Purchio A. F., Bursch W., Schulte-Hermann R. Induction of apoptosis in cultured hepatocytes and in regressing liver by transforming growth factor beta 1. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5408–5412. doi: 10.1073/pnas.89.12.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhammer F., Bursch W., Parzefall W., Breit P., Erber E., Stadler M., Schulte-Hermann R. Effect of transforming growth factor beta on cell death of cultured rat hepatocytes. Cancer Res. 1991 May 1;51(9):2478–2485. [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Wakefield L. M., Roche N. S., Stern D. F., Sporn M. B. Type beta transforming growth factor: a bifunctional regulator of cellular growth. Proc Natl Acad Sci U S A. 1985 Jan;82(1):119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. D., Roberts A. B., Daniel C. W. TGF beta suppresses casein synthesis in mouse mammary explants and may play a role in controlling milk levels during pregnancy. J Cell Biol. 1993 Jan;120(1):245–251. doi: 10.1083/jcb.120.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. D., Silberstein G. B., Roberts A. B., Flanders K. C., Daniel C. W. Regulated expression and growth inhibitory effects of transforming growth factor-beta isoforms in mouse mammary gland development. Development. 1991 Nov;113(3):867–878. doi: 10.1242/dev.113.3.867. [DOI] [PubMed] [Google Scholar]
- Samuel S. K., Hurta R. A., Kondaiah P., Khalil N., Turley E. A., Wright J. A., Greenberg A. H. Autocrine induction of tumor protease production and invasion by a metallothionein-regulated TGF-beta 1 (Ser223, 225). EMBO J. 1992 Apr;11(4):1599–1605. doi: 10.1002/j.1460-2075.1992.tb05205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay A., Solinas S., Pursel V. G., McKnight R. A., Alexander L., Beattie C., Hennighausen L., Wall R. J. Production of the mouse whey acidic protein in transgenic pigs during lactation. J Anim Sci. 1991 Nov;69(11):4552–4562. doi: 10.2527/1991.69114552x. [DOI] [PubMed] [Google Scholar]
- Shull M. M., Ormsby I., Kier A. B., Pawlowski S., Diebold R. J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992 Oct 22;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein G. B., Daniel C. W. Reversible inhibition of mammary gland growth by transforming growth factor-beta. Science. 1987 Jul 17;237(4812):291–293. doi: 10.1126/science.3474783. [DOI] [PubMed] [Google Scholar]
- Silberstein G. B., Flanders K. C., Roberts A. B., Daniel C. W. Regulation of mammary morphogenesis: evidence for extracellular matrix-mediated inhibition of ductal budding by transforming growth factor-beta 1. Dev Biol. 1992 Aug;152(2):354–362. doi: 10.1016/0012-1606(92)90142-4. [DOI] [PubMed] [Google Scholar]
- Smith G. H., Gallahan D., Zwiebel J. A., Freeman S. M., Bassin R. H., Callahan R. Long-term in vivo expression of genes introduced by retrovirus-mediated transfer into mammary epithelial cells. J Virol. 1991 Nov;65(11):6365–6370. doi: 10.1128/jvi.65.11.6365-6370.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. H., Medina D. A morphologically distinct candidate for an epithelial stem cell in mouse mammary gland. J Cell Sci. 1988 May;90(Pt 1):173–183. doi: 10.1242/jcs.90.1.173. [DOI] [PubMed] [Google Scholar]
- Smith G. H., Mehrel T., Roop D. R. Differential keratin gene expression in developing, differentiating, preneoplastic, and neoplastic mouse mammary epithelium. Cell Growth Differ. 1990 Apr;1(4):161–170. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Transforming growth factor-beta. Multiple actions and potential clinical applications. JAMA. 1989 Aug 18;262(7):938–941. doi: 10.1001/jama.262.7.938. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Transforming growth factor-beta: recent progress and new challenges. J Cell Biol. 1992 Dec;119(5):1017–1021. doi: 10.1083/jcb.119.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange R., Li F., Saurer S., Burkhardt A., Friis R. R. Apoptotic cell death and tissue remodelling during mouse mammary gland involution. Development. 1992 May;115(1):49–58. doi: 10.1242/dev.115.1.49. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield L. M., Winokur T. S., Hollands R. S., Christopherson K., Levinson A. D., Sporn M. B. Recombinant latent transforming growth factor beta 1 has a longer plasma half-life in rats than active transforming growth factor beta 1, and a different tissue distribution. J Clin Invest. 1990 Dec;86(6):1976–1984. doi: 10.1172/JCI114932. [DOI] [PMC free article] [PubMed] [Google Scholar]