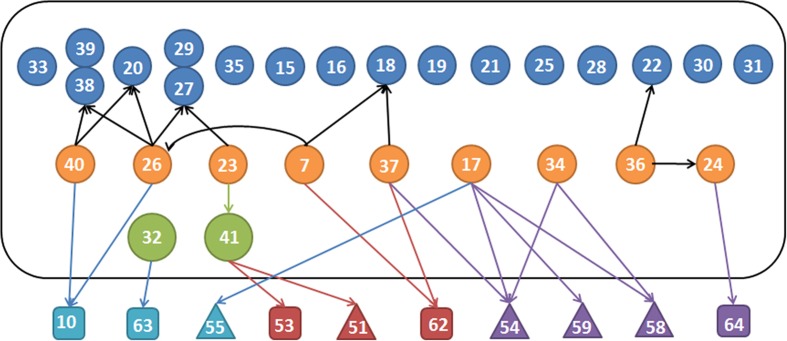
Fig. 1.
Publications linked to the references they cite to underpin this guidance. Circles within the black rectangle: the 28 publications found to state this guidance. By colour: blue, without supporting citations; orange, provide non-relevant supporting citations; green, provide relevant supporting citations. Outside the rectangle, in squares, cited human publications that are not relevant: in light blue, primary OP pesticide papers; in red, primary cardiac disease papers; in purple, primary nerve agent papers. Triangles: animal studies. Numbers correspond with the references. Publications from the same book, with a different edition, are placed together (refs 27 and 29, and 38 and 39). Of the 28 publications, 11 cited a source for their statement while 17 did not provide any supporting evidence. The 11 publications cited 18 sources on 27 occasions. Eleven citations were to relevant but secondary sources18,20,22,24,26,29,38 while seven citations were of irrelevant primary or secondary sources (patients with myocardial infarctions, nerve agent studies, or patients with pesticide poisoning but no ventricular dysrhythmias10,53,62–64). Animal studies with OP nerve agents, pesticide active ingredients, or myocardial infarctions51,54,55,58,59 were cited on eight occasions (colour version of this figure can be found in the online version at www.informahealthcare.com/ctx).