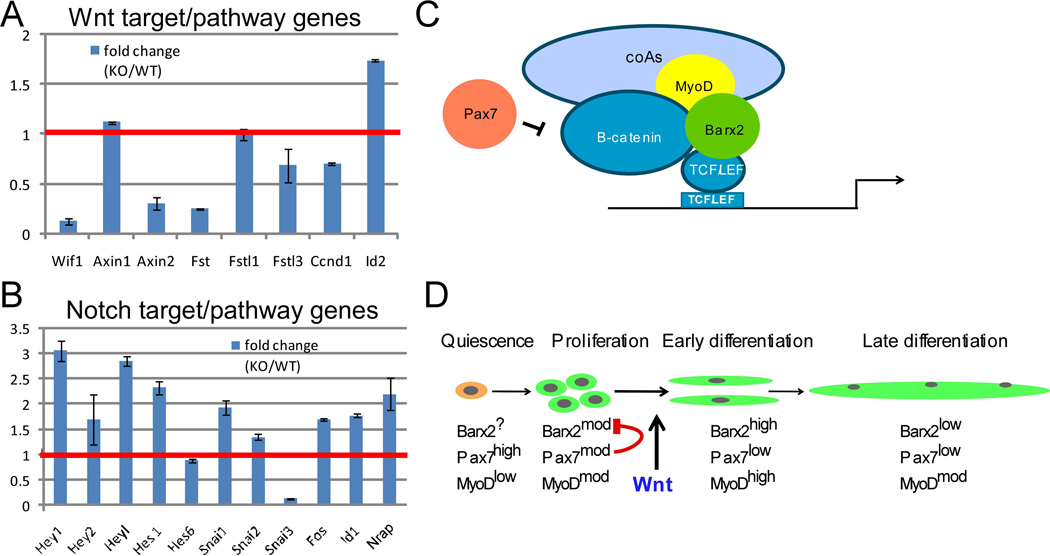
Figure 5.
A, B. Relative expression of a representative set of Wnt and Notch target and pathway genes in Barx2+/+ (WT) and Barx2−/− (KO) primary myoblasts obtained from RNA-seq analysis. The red line denotes the level in Barx2+/+ (WT) myoblasts which is set to 1. All gene expression changes greater than 1.5 fold are significant at P<0.05 (see also Supplemental Table S1). C. Model of the transcriptional complexes identified in this study. Barx2 interacts with β-catenin and TCF/LEF factors as well as MyoD. This complex recruits co-activators that promote transcription via TCF/LEF sites. Pax7 inhibits the function of this complex, likely via interaction with β-catenin which could lead to sequestering of β-catenin, and/or failure to dismiss co-repressors and recruit coactivators. D. Barx2 and Pax7 may control the switch from proliferation to differentiation via antagonistic regulation of Wnt signaling. In proliferating myoblasts both Barx2 and Pax7 are expressed at moderate levels, thus Pax7 may inhibit the ability of Barx2 to activate via β-catenin-TCF/LEF complexes. After induction of differentiation by canonical Wnt signals, Barx2 is upregulated and Pax7 is downregulated. This may allow Barx2 to activate TCF/LEF target genes associated with early differentiation events. Later in mature myofibers, expression of both Barx2 and Pax7 is low or absent.