Figure 1.
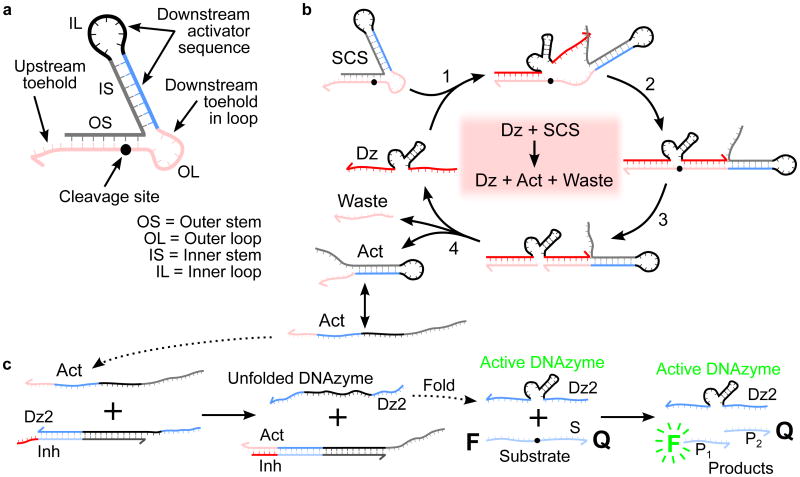
SCS design and mechanisms for SCS cleavage and DNAzyme displacement. a) Design of a structured chimeric substrate (SCS) to enable signaling between DNAzymes. The SCS consists of an outer stem and loop, which make up the upstream DNAzyme binding domain (red), and an inner stem and loop, which sequester a downstream activator sequence (blue and black). The cleavage site is located towards the inner end of the outer stem. The grey cage sequence is chosen to fold into the desired structure, producing a topological constraint on the downstream reaction kinetics that is undone when the SCS is cleaved by the upstream DNAzyme. b) Mechanism of cleavage of the SCS by an upstream DNAzyme (Dz). The upstream DNAzyme binds to the outer stem and loop by toehold-mediated strand displacement. The cleavage reaction produces a waste strand and an activator strand (Act). In the activator structure, the outer loop has been released from the topological constraint previously imposed by the outer stem, making the downstream toehold in the outer loop available to bind with a downstream circuit element. c) DNAzyme displacement reaction mechanism. The catalytic activity of the downstream DNAzyme strand (Dz2) is inhibited by hybridization to a partially complementary inhibitor strand (Inh) with a short overhanging toehold. Activation is by a toehold-mediated strand displacement reaction: an input strand (Act) binds to the complex (Dz2-Inh) via the toehold. The input initiates a branch-migration reaction that eventually displaces a catalytically active downstream DNAzyme strand (Dz2), leaving an inert waste complex (Act-Inh). The DNAzyme strand then folds into a catalytically active conformation and proceeds to bind to substrate molecules (S) and cleave them, producing shorter cleavage products (P1 and P2). The cleavage reaction causes separation of the fluorophore-quencher pair attached to the two ends of the substrate, observed as an increase in bulk fluorescence due to loss of FRET.