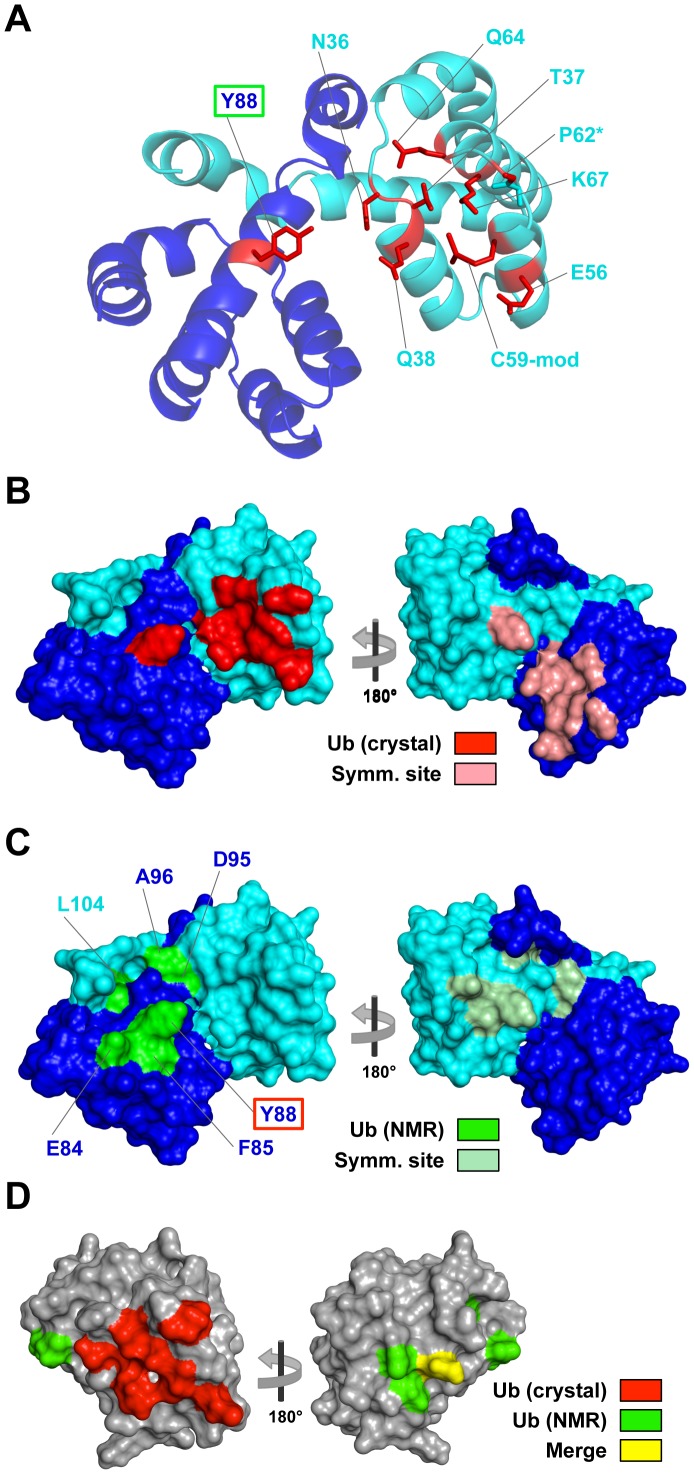
Figure 4. Ub interaction sites on NOD1 CARD.
A) The NOD1 CARD dimer from our crystal structure (CARD A, cyan and CARD B, blue) is depicted in cartoon view. Residues mediating interaction with Ub (red) are shown as sticks. The asterisk on Pro62 indicates that only the main chain of this residue is involved in the interaction. The Cys59 sulfhydryl is modified with a dimethyl-As adduct (C59-mod – see figure 4). The green box around Tyr88 signifies that this residue is also involved in the previously identified NMR Ub-binding site. B) Surface views of the NOD1 CARD dimer showing the Ub binding site in the crystal. The majority of the binding site is on CARD A in the asymmetric unit, however Tyr88 from the CARD B subunit of the dimer is also involved in the interaction. The symmetrical binding site on the opposite side of the dimer is shown (pink) on the rotated view. C) Surface views of the NOD1 CARD dimer showing the Ub binding site previously identified by NMR (green and light green). This site is located primarily on helix α5, which is on a different surface of NOD1 CARD than the one in the crystal structure. In the dimer, the two sites occupy the same overall surface formed from both subunits, while in the monomer they are on different surfaces (see D). Due to helix swapping in the dimer, the Leu104 sidechain is derived from helix 6 on the opposite subunit. The red box around Tyr88 signifies that it is also involved in the Ub-binding site observed in the crystal. D) Surface views of the NOD1 CARD monomer (PDB ID: 2DBD) with both Ub-binding sites mapped onto it. This demonstrates that the sites occupy different surfaces on the monomer, with the implication that the full interaction observed in the crystal could not occur with the monomeric form of NOD1 CARD unless Tyr88 was not involved. This arrangement of two different binding sites on two different surfaces is similar to that seen in the structure of the HOIL-1L NZF domain bound to linear di-Ub [34].