Abstract
Objective
We prospectively evaluated the Haptoglobin (Hp)-stroke association in type 1 diabetes and hypothesized that despite increasing the risk for coronary artery disease, the presence of the Hp 2 allele would lower stroke incidence.
Methods
Participants from the Epidemiology of Diabetes Complications study without prevalent stroke and Hp available were evaluated (n=607; mean age, 27.6 and duration, 19.3 years).
Results
During 22 years of follow-up, stroke incidence did not differ by Hp genotype (p=0.49). Restricting analyses to those diagnosed with diabetes ≥1965 (13% mortality versus 40% in the <1965 cohort) to diminish potential survival bias, the adjusted HR for Hp 1-1 was 3.08 (95% CI=0.81-11.77, p=0.10). Further stratifying by hypertension prevalence, an increased stroke incidence was observed with Hp 1-1 only in those with hypertension (HR=7.03, 95% CI=1.42-34.89, p=0.02).
Conclusions
Despite the protective effect against vascular diabetes complications, a borderline increased risk for stroke was observed with Hp 1-1 in type 1 diabetes. This mixed Hp effect on cardiovascular risk by outcome studied merits further investigation and cautions against the universal application of preventive therapies across all Hp genotypes.
Keywords: Type 1 diabetes, stroke, coronary artery disease, haptoglobin genotype, hypertension
Introduction
Data on cerebrovascular disease in type 1 diabetes are scarce. The few existing reports suggest that, as in the general population, stroke in type 1 diabetes is largely ischaemic and usually not preceded by either coronary artery disease (CAD) or transient ischaemic attack (1-4). However, stroke incidence is highly increased in type 1 diabetes and occurs at least 20 years earlier than in the general population (1). Moreover, survival following an incident event is remarkably low and estimated at 45% five years after stroke (1).
Diabetes duration has been shown to be the strongest predictor of stroke in type 1 diabetes (1), although modifiable risk factors such as poor glycemic control, hypertension and dyslipidemia were also shown to play a role (1-2, 4). Recently, studies in the general population showed a lower prevalence of the Haptoglobin (Hp) 2 allele among individuals with cerebrovascular disease (5-7), a surprising observation given consistent prospective findings of an increased cardiovascular and end-stage renal disease (8) risk associated with the Hp 2 allele in diabetes.
A major role of Hp, an acute phase α2-glycoprotein, is to bind free hemoglobin and remove it from circulation either with the aid of hepatocytes or by attaching to CD163 monocyte/macrophage scavenger receptor (9). This hemoglobin binding property of Hp has led to its classification as an antioxidant, since it serves to inhibit heme iron release and, thus, reduces hemoglobin-induced oxidative tissue damage (9). In humans, two classes of alleles exist at the Hp locus (the 5-exon class 1 allele and the class 2 allele, which arose from duplication of exons 3 and 4 of the Hp 1 allele), giving rise to three genotypes: Hp 1-1, Hp 2-1 and Hp 2-2 (9). The Hp 1 protein allele has been described as having greater antioxidant capacity (10-12) and inflammatory down-regulation activity (13). Conversely, although Hp 2 has greater angiogenic potential (9), it has been associated with impaired HDL function (14). These functional differences, which are thought to be exaggerated in the presence of diabetes (8), may potentially explain the evidence linking the Hp genotype to vascular complication outcomes only in those with diabetes or in the presence of hyperglycemia (15-17).
It is also important to note that although numerous trials have shown no, or even a harmful, effect of antioxidant treatment in terms of cardiovascular outcomes, three clinical trials have provided evidence for a protective effect of vitamin E therapy against CVD among individuals with diabetes and the Hp 2-2 genotype (18-20). Taken together, these findings suggest an adverse effect of vitamin E therapy in carriers of the Hp 1 allele, which could be due to exacerbation of a potential stroke susceptibility in the Hp 1-1 group, should findings in the general population (5-7) hold true also in diabetes. To our knowledge, however, such assessments in diabetes have yet to be conducted. We, thus, aimed to prospectively evaluate the presence of an association between the Hp genotype and incident cerebrovascular disease in a type 1 diabetes cohort, hypothesizing that, as in the general population, the Hp 2 allele would be inversely related to stroke incidence.
Research Design and Methods
The EDC study was based on a historical cohort of incident, childhood-onset (<17 years), type 1 diabetes cases, diagnosed or seen within one year of diagnosis (1950-1980) at Children’s Hospital of Pittsburgh. This cohort has been shown to be representative of the Allegheny County, Pennsylvania, type 1 diabetes population (21). The first participant assessment occurred in 1986-1988, when mean participant age and duration of diabetes were 28 and 19 years, respectively. Subsequently, biennial examinations were conducted for 10 years and additionally at 18 years. Demographic, health-care, diabetes self-care and medical history information was assessed using questionnaires. The University of Pittsburgh IRB approved the study protocol.
Weight was measured on a balance beam scale with participants barefoot and in light clothing and height using a stadiometer. Body mass index (BMI) was calculated as weight (kg) divided by height (m2). Waist to hip ratio was calculated as the ratio of waist (measured at the midpoint between the highest point of the iliac crest and the lowest part of the costal margin in the midaxillary line) to hip (measured at the widest point of the glutei, usually at the level of the greater femoral trochanter) girth. An ever smoker was defined as a person who had smoked ≥100 cigarettes over their lifetime. Blood pressure was measured with a random zero sphygmomanometer after a five minute rest (22) and hypertension was defined as ≥130/80 mmHg or antihypertensive medication use. Stroke incidence was determined through biennial surveys or physician interviews. When possible, medical or autopsy records were obtained to verify the occurrence and type of stroke. Confirmatory medical or autopsy records were obtained for 77% of the events. Stroke was defined as a neurological deficit of acute onset lasting ≥24 hours without other evident cause.
Stable glycosylated hemoglobin (HbA1) was measured by ion exchange chromatography (Isolab, Akron, OH) and subsequently by automated high-performance liquid chromatography (Diamat, BioRad, Hercules, CA). The two assays were highly correlated (r=0.95). HDL cholesterol was determined by a precipitation technique (23). Cholesterol and triglycerides were measured enzymatically (24-25). Non-HDL was calculated as total minus HDL cholesterol. White blood cell count was obtained using a counter S-plus IV. Urinary albumin was measured by immunonephelometry (26) and creatinine was assayed by an Ectachem 400 Analyzer (Eastman Kodak Co., Rochester, NY). Glomerular filtration rate (eGFR) was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI) (27). For 486 participants who provided DNA, Hp was genotyped by an amplification method (28) as previously described (29) while for 129 participants without DNA but with stored blood samples available, Hp phenotype was assessed using an Elisa test (30). Of 615 participants with DNA available, four individuals (one Hp 1-1, one Hp 2-1 and two Hp 2-2) with prevalent cerebrovascular disease and an additional four missing clinical information were excluded from the analyses.
Statistical analysis
Univariate associations were determined using parametric or non-parametric tests for continuous variables and the Χ2 or Fisher’s exact test for categorical variables, as appropriate. Cox proportional hazards models with backward elimination were constructed to assess the presence of an independent association between the Hp genotype and cerebrovascular disease incidence. Models allowed for baseline variables found to be univariately (crudely) associated with cerebrovascular disease incidence (diabetes duration in years, having ever smoked (% yes/no), HbA1c (%), hypertension (% yes/no), HDL cholesterol (mg/dL), non-HDL cholesterol (mg/dL), albumin excretion rate (μg/min), eGFR (mL/min/1.73 m2) and white blood cell count (x 103/mm2)) and were based on participants with complete data on all covariates included in the models. Survival time was defined as the time in years from study entry to an incident event or censorship during the 22 year follow-up. Hazard ratios were reported per one unit increment for continuous variables. Statistical analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC).
Results
During 22 years of follow-up, incident cerebrovascular disease occurred in 5.4% (33/607). Table 1 presents baseline demographic and clinical participant characteristics by subsequent stroke occurrence. As expected, incident cases were more likely to have been older, with longer diabetes duration, to have ever smoked and to have hypertension. Incident cases also had higher concentrations of non-HDL cholesterol, white blood cell count and albumin excretion rate and lower eGFR. No differences were observed in stroke incidence by gender or race/ethnicity; however, as the majority of study participants (98%) were non-Hispanic, no conclusions can be drawn from this study on the association between race/ethnicity and cerebrovascular disease incidence in type 1 diabetes.
Table 1.
Baseline participant characteristics by stroke incidence (n=607)
| No Stroke (n=574) | Stroke (n=33)* | p-value | |
|---|---|---|---|
| Age (years) | 27.2 (7.8) | 33.4 (7.3) | <0.0001 |
| Age at onset (years) | 8.3 (4.1) | 7.8 (4.0) | 0.50 |
| Duration (years) | 19.0 (7.5) | 25.6 (6.5) | <0.0001 |
| Follow-up (years) | 18.6 (5.8) | 9.5 (7.0) | <0.0001 |
| Females (%, n) | 48.1 (276) | 48.5 (16) | 0.96 |
| Race / Ethnicity (%, n) | |||
| Non-Hispanic white | 97.9 (562) | 100.0 (33) | |
| African-American | 2.1 (12) | 0.0 (0) | 1.00 |
| Body mass index (kg/m2, n=572;33) | 23.6 (3.2) | 23.9 (3.3) | 0.57 |
| Waist to hip ratio (n=569;33) | 0.82 (0.07) | 0.85 (0.09) | 0.15 |
| Smoking, ever (%, n) | 36.1 (207) | 54.5 (18) | 0.03 |
| HbA1c (%) | 8.7 (1.5) | 9.0 (1.7) | 0.38 |
| HbA1c (mmol/mol) | 72 | 75 | |
| Systolic blood pressure (mmHg) | 112.8 (15.3) | 126.1 (18.0) | <0.0001 |
| Diastolic blood pressure (mmHg) | 72.4 (10.8) | 78.2 (13.7) | 0.02 |
| Hypertension (%, n) | 29.3 (168) | 57.6 (19) | 0.0006 |
| HDL cholesterol (mmol/L, n=571;32) | 1.4 (0.3) | 1.3 (0.3) | 0.49 |
| Non-HDL (mmol/L, n=571;32) | 3.5 (1.1) | 4.2 (1.2) | <0.0001 |
| Albumin excretion rate
(μg/min, n=570;32) |
14.1 (7.2-104.4) | 377.5 (17.8-1186.3) | <0.0001 |
| eGFR (mL/min/1.73 m2, n=573;33) | 103.2 (31.8) | 80.6 (37.0) | <0.0001 |
| White blood cell count ×
103/mm2 (n=570;33) |
6.5 (1.8) | 7.6 (2.4) | 0.01 |
9 hemorrhagic and 23 ischemic strokes
Generally, with the exception of younger age among those with the Hp 2-2 compared to the Hp 2-1 genotype, participant characteristics did not differ by Hp genotype (Supplemental Table S1). A non-significant trend toward lower systolic blood pressure from the Hp 1-1 to the Hp 2-2 genotype was, however, noted.
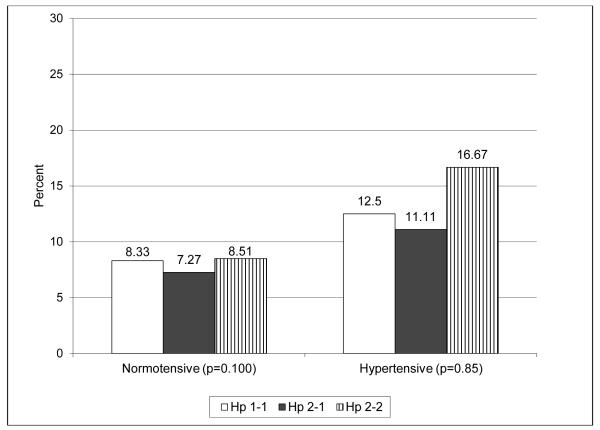
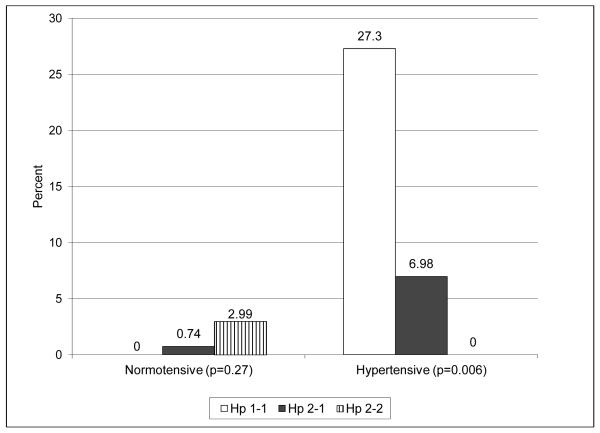
Overall, the proportion of incident stroke cases did not differ by Hp genotype or Hp allele frequency (Table 2). To examine the possibility that mortality prior to an individual’s baseline assessment affected the distribution of the Hp genotype in this cohort and that survival bias has distorted the true association between Hp and stroke incidence, we conducted stratified analyses by diabetes diagnosis year before or after 1965. This cut off point was used given available data suggesting that 40% of those diagnosed with type 1 diabetes prior to 1965 and eligible to partake in the EDC study died before the baseline assessment whereas only 13% of those diagnosed after 1965 died before study initiation. Although no significant differences were observed in stroke incidence by Hp in either diagnosis cohort, rates were three times higher with Hp 1 homozygosity in the more recent diagnosis cohort (p=0.27). Combining carriers of any Hp 2 allele did not alter findings. When analyses were repeated further stratifying the two cohorts by hypertension status (Figure 1), similar rates were observed by Hp in the earlier diagnosis cohort regardless of hypertension status (Figure 1A). However, incidence was significantly increased with Hp 1-1 in those diagnosed ≥1965 who had hypertension (Figure 1B, p=0.006), while no differences were observed by Hp genotype among normotensive individuals.
Table 2.
Strokeincidence by haptoglobin genotype (n=607)
| Incidence of stroke (%) | |||
|---|---|---|---|
| Diabetes diagnosed | |||
| Overall cohort | <1965 | >1965 | |
| Hp genotype | |||
| 1-1 | 7.9 (6/76) | 10.7 (3/28) | 6.2 (3/48) |
| 2-1 | 4.7 (13/278) | 9.1 (9/99) | 2.2 (4/179) |
| 2-2 | 5.5 (14/253) | 12.3 (10/81) | 2.3 (4/172) |
| p-value: | 0.49* | 0.78* | 0.27* |
| p-trend: | 0.67 | 0.64 | 0.27 |
| Hp genotype | |||
| 1-1 | 7.9 (6/76) | 10.7 (3/28) | 6.2 (3/48) |
| 2-1/2-2 | 5.1 (27/531) | 10.6 (19/180) | 2.3 (8/351) |
| p-value: | 0.29* | 1.00* | 0.13* |
| Hp alleles | |||
| Hp 1 | 5.8 (25/430) | 9.7 (15/155) | 3.6 (10/275) |
| Hp 2 | 5.2 (41/784) | 11.1 (29/261) | 2.3 (12/523) |
| p-value: | 0.67 | 0.65 | 0.27 |
Fisher’s exact test
Figure 1.
Stroke incidence by Hp genotype, hypertension status, and diagnosis cohort
A. Diabetes diagnosis <1965. White bars = Hp 1-1; black bars = Hp 2-1; bars with stripes = Hp 2-2.
B. Diabetes diagnosis ≥1965. White bars = Hp 1-1; black bars = Hp 2-1; bars with stripes = Hp 2-2.
We further evaluated the participants’ age at the time the incident stroke event occurred by Hp genotype. Compared to incident cases carrying at least one Hp 2 allele, those homozygous for Hp 1 were younger at stroke occurrence (36.8 vs. 44.2 yrs, p=0.06).
In assessing differences in the type of cerebrovascular event by Hp genotype, we noted that although the incidence of both ischemic and hemorrhagic stroke appeared higher with the Hp 1-1 genotype compared to having at least one Hp 2 allele, results did not reach statistical significance (p-value=0.73 and 0.51 for ischemic and hemorrhagic stroke, respectively). Comparing Hp 1-1 versus the presence of any Hp 2 allele did not alter these findings (p-value=0.52 and 0.31 for ischemic and hemorrhagic stroke, respectively).
In multivariable Cox models, the increased risk associated with Hp 1 allele homozygosity did not reach significance in the overall cohort, whereas diabetes duration, non-HDL cholesterol and albumin excretion rate directly predicted stroke incidence (Table 3). In models stratifying by diabetes diagnosis year, Hp 1-1 was associated with over a threefold increased risk of stroke (p=0.10) in the most recent and a non-significant 27% risk reduction in the earlier diagnosis cohort. Other factors that were directly related to stroke incidence in the most recent diagnosis cohort were duration of diabetes, HbA1c, hypertension whereas in the earlier diagnosis cohort significant predictors comprised non-HDL cholesterol and albumin excretion rate (having ever smoked, HDL cholesterol, eGFR and white blood cell count were not significantly related to stroke incidence in any analysis). Further stratifying analyses by hypertension prevalence suggested a significantly increased stroke risk with Hp 1-1 (HR=7.03, 95% CI=1.42-34.89, p=0.02) for the ≥1965 diagnosis cohort with hypertension, while no incident events occurred among Hp 1-1 carries in normotensive individuals. However, assessing whether the effect of Hp differed by hypertension status in a model that included an interaction term (Hp*hypertension, p=0.99) in addition to lower order terms, did not confirm the presence of effect modification, though this was likely due to the small sample size. Repeating all multivariable analyses adjusting for age instead of diabetes duration did not significantly alter results.
Table 3.
Cox models* for the prediction of incident stroke
| Total cohort (n=594; 31 events) |
Diagnosed <1965 (n=201; 20 events) |
Diagnosed ≥1965 (n=393; 11 events) |
|
|---|---|---|---|
| Haptoglobin | |||
| 1-1 versus 2-1/2-2 | 1.38 (0.53-3.63) | 0.73 (0.17-3.17) | 3.08 (0.81-11.77)† |
| Years of diabetes duration | 1.11 (1.05-1.17) | NS | 1.23 (1.03-1.47) |
| HbA1c (%) | NS | NS | 1.39 (0.96-1.99) |
| Hypertension | |||
| Yes vs. No | NS | NS | 3.21 (0.93-11.05) |
| Non-HDL cholesterol (mg/dL) | 1.01 (1.00-1.02) | 1.01 (1.00-1.02) | NS |
| Albumin excretion
rate (μg/min) |
1.32 (1.10-1.58) | 1.47 (1.17-1.85) | NS |
Data presented are hazard ratios (95% CI). Hazard ratios are presented per 1 unit increment for continuous variables (diabetes duration, HbA1c, non-HDL cholesterol and albumin excretion rate). Models also allowed for: ever smoker, HDL cholesterol, eGFR, and white blood cell count. NS: Not selected.
p-value = 0.10
Conclusions
In this cohort of long duration, childhood-onset, type 1 diabetes, we failed to see any protection against cerebrovascular disease with Hp 1 allele homozygosity, and indeed observed a borderline significant increased risk. This observation was particularly apparent in the cohort more recently (after 1965) diagnosed with type 1 diabetes, where survival bias was less likely to have affected the distribution of the Hp genotype and Hp’s association with stroke occurrence. In this sub-cohort, the increased risk of stroke associated with the Hp 1-1 genotype appeared restricted to those with hypertension.
Our present findings are in striking contrast to the previously reported prospective associations between the Hp genotype and incident cardiovascular disease in both type 1 (16, 29) and type 2 diabetes (30-33) or hyperglycemia (15), where the Hp 2-2 genotype was shown to confer greater disease risk. However, earlier studies did not specifically evaluate the Hp-stroke association. Interestingly, in type 1 diabetes, the number of Hp 2 alleles has also been associated with a linearly increased risk of decreased kidney function in the present population (34), a finding which was recently replicated within the DCCT/EDIC study (35).
The elevated risk of cardiovascular and kidney complications associated with the Hp 2 allele in diabetes has generally not been observed in the general population. Indeed, a recent analysis (15) confirmed the presence of effect modification in the Hp - coronary heart disease risk association by increased glucose levels. Thus, the Hp 2-2 genotype was significantly associated with increased coronary heart disease risk only in those with HbA1c levels above 6.5%.
Notwithstanding the lack of an association with coronary heart disease or kidney function in the general population, recent data from Europe suggest a direct relationship between the Hp 1 allele frequency and stroke, consistent with our results in type 1 diabetes. Specifically, compared to healthy volunteers, a greater frequency of the Hp 1 allele was found among patients with a first symptomatic small vessel lacunar stroke and Hp 1 was also related to white matter lesions (5). Similarly to our findings, the direct effect of the Hp 1 allele appeared more pronounced among those with hypertension, while an association was absent in normotensive individuals. Interestingly, Hp 1-1 patients were more likely to have hypertension, and the authors suggested the presence of high blood pressure as one potential mechanism by which the Hp 1 allele related to lacunar stroke, given the importance of hypertension in the pathophysiology of cerebral small vessel disease.
In our type 1 diabetes population, the prevalence of hypertension did not differ by Hp genotype although systolic blood pressure increased slightly with the number of Hp 1 alleles. Whether the Hp 1 allele is generally associated with hypertension, however, is unclear. Indeed, reports of both elevated blood pressure readings with Hp 1 allele homozygosity (36) and no difference by Hp (35, 37) have been published. Interestingly, previous research also suggested that among patients with hypertension, the Hp 2-2 genotype is associated with a requirement for more complex antihypertensive regimen; the presence of more severe vascular complications (38); and an increased risk of developing refractory hypertension compared to other Hp phenotypes (39). Whether these associations relate to our observation that the increased Hp 1-1 stroke risk is seen only in those with hypertension, is unclear.
In a separate study of 42 lacunar stroke cases (6), patients with Hp 1-1 were shown to have impaired endothelial progenitor cell cluster formation compared to carriers of at least one Hp 2 allele. In vitro experiments conducted by these investigators provided further data that the addition of Hp 1-1 to endothelial progenitor cell clusters, produced lower cluster numbers than the addition of other Hp types, suggesting that Hp 1 may relate to cerebral small vessel disease via its decreased endothelial repair potential (6). Indeed, among 152 hypertensive patients without symptomatic vascular disease, carrying the Hp 1-1 type was associated with larger deep cerebral white matter lesion volumes but not with periventricular white matter lesion volumes (7). As the majority of patchy and diffuse white matter lesions in the deep white matter are thought to relate to cerebrovascular disease, it was hypothesized that this Hp 1-1 effect may be attributed to a lower regenerating power of the Hp 1 allele against endothelial injury.
As previously proposed (5, 7), it is indeed conceivable that functional differences across Hp genotypes are responsible for these discrepancies in its association with atherosclerosis or kidney dysfunction versus cerebrovascular disease. Thus, excessive angiogenesis, more of a characteristic of Hp 2-2 than Hp 1-1 (9), may lead to increased atherosclerosis and kidney disease risk, but be relatively protective for cerebrovascular complications. Conversely, the greater antioxidant and anti-inflammatory capacity of Hp 1-1 (9) may account for the observed protection it confers against CAD and kidney dysfunction. Furthermore, the diverse effects of Hp genotype on CAD and cerebrovascular disease may relate to the observation that vitamin E protects against CAD only in those with diabetes carrying the Hp 2 allele but may have little or even a harmful effect in the non-Hp 2-2 individuals (18-20, 40). Administration of vitamin E may thus exacerbate the risk of stroke, especially in the Hp 1-1 group.
A major limitation of the present study is the small number of events, which prohibited a more refined classification of type of cerebrovascular event and assessment of the role of Hp specifically on lacunar strokes, as previously suggested in the general, non-diabetes, population. A strength, however, is the evaluation of this association prospectively in a generally young type 1 diabetes population. Nevertheless, even in this cohort of relatively young adults with type 1 diabetes, survival bias could not be excluded, although this was addressed by stratified analysis by diabetes diagnosis year.
In conclusion, despite the previously reported protective effect against CAD and kidney dysfunction, no protection and even an increased risk of cerebrovascular disease, particularly in those with hypertension, was observed with the Hp 1-1 genotype in this childhood-onset type 1 diabetes cohort. This mixed effect of the Hp genotype on cardiovascular risk according to outcome studied in diabetes merits further investigation and underscores the need for caution in the universal application of preventive therapies across all Hp genotypes.
Supplementary Material
Acknowledgments
T.C. researched/analyzed the data and wrote the manuscript; A.M.S. researched data and reviewed/edited the manuscript; R.E.F. assessed Hp genotype, and reviewed/edited the manuscript; T.J.O. researched data, contributed to the discussion and reviewed/edited the manuscript. The authors declare no conflicts of interest related to this research. All authors have read and approved the final version of the manuscript. As the corresponding author and guarantor of this article, T.C. takes full responsibility for the work as a whole. We thank study participants and the EDC study staff for their invaluable contributions. This research was supported by NIH grant DK34818 and the Rossi Memorial Fund. Preliminary data were presented at the International Stroke Conference, February 2013, Honolulu, Hawaii.
References
- 1.Secrest AM, Prince CT, Costacou T, Miller RG, Orchard TJ. Predictors of and survival after incident stroke in type 1 diabetes. Diab Vasc Dis Res. 2013;10:3–10. doi: 10.1177/1479164112441006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuller JH, Stevens LK, Wang SL. Risk factors for cardiovascular mortality and morbidity: the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(Suppl 2):S54–S64. doi: 10.1007/pl00002940. [DOI] [PubMed] [Google Scholar]
- 3.Laing SP, Swerdlow AJ, Carpenter LM, et al. Mortality from cerebrovascular disease in a cohort of 23 000 patients with insulin-treated diabetes. Stroke. 2003;34:418–421. doi: 10.1161/01.str.0000053843.03997.35. [DOI] [PubMed] [Google Scholar]
- 4.Davis TM, Bruce DG, Davis WA. Predictors of first stroke in Type 1 diabetes: The Fremantle Diabetes Study. Diabet Med. 2005;22:551–553. doi: 10.1111/j.1464-5491.2005.01462.x. [DOI] [PubMed] [Google Scholar]
- 5.Staals J, Pieters BMA, Knottnerus ILH, Rouhl RPW, van Oostenbrugge RJ, Delanghe JR, Tervaert JW, Lodder J. Haptoglobin Polymorphism and Lacunar Stroke. Curr Neurovasc Res. 2008;5:153–158. doi: 10.2174/156720208785425675. [DOI] [PubMed] [Google Scholar]
- 6.Rouhl RP, van Oostenbrugge RJ, Damoiseaux JG, Debrus-Palmans LL, Theunissen RO, Knottnerus IL, Staals JE, Delanghe JR, Tervaert JW, Lodder J. Haptoglobin phenotype may alter endothelial progenitor cell cluster formation in cerebral small vessel disease. Curr Neurovasc Res. 2009;6:32–41. doi: 10.2174/156720209787466082. [DOI] [PubMed] [Google Scholar]
- 7.Staals J, Henskens LHG, Delanghe JR, van Oostenbrugge RJ, Kessels AG, Kroon AA, de Leeuw PW, Lodder J. Haptoglobin Phenotype Correlates with the Extent of Cerebral Deep White Matter Lesions in Hypertensive Patients. Curr Neurovasc Res. 2010;7:1–5. doi: 10.2174/156720210790820163. [DOI] [PubMed] [Google Scholar]
- 8.Costacou T, Levy AP. Haptoglobin genotype and its role in diabetic cardiovascular disease. J Cardiovasc Transl Res. 2012;5:423–435. doi: 10.1007/s12265-012-9361-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem. 1996;42:1589–1600. [PubMed] [Google Scholar]
- 10.Asleh R, Marsh S, Shilkrut M, Binah O, Guetta J, Lejbkowicz F, Enav B, Shehadeh N, Kanter Y, Lache O, Cohen O, Levy NS, Levy AP. Genetically determined heterogeneity in hemoglobin scavenging and susceptibility to diabetic cardiovascular disease. Circ Res. 2003;92:1193–1200. doi: 10.1161/01.RES.0000076889.23082.F1. [DOI] [PubMed] [Google Scholar]
- 11.Asleh R, Guetta J, Kalet-Litman S, Miller-Lotan R, Levy AP. Haptoglobin genotype and diabetes dependent differences in iron mediated oxidative stress in vitro and in vivo. Circ Res. 2005;96:435–441. doi: 10.1161/01.RES.0000156653.05853.b9. [DOI] [PubMed] [Google Scholar]
- 12.Levy AP, Purushothaman KR, Levy NS, Purushothaman M, Strauss M, Asleh R, Marsh S, Cohen O, Moestrup SK, Moller HJ, Zias EA, Benhayon D, Fuster V, Moreno PR. Downregulation of the Hemoglobin Scavenger Receptor in Individuals With Diabetes and the Hp 2-2 Genotype: implications for the response to intraplaque hemorrhage and plaque vulnerability. Circ Res. 2007;101:106–110. doi: 10.1161/CIRCRESAHA.107.149435. [DOI] [PubMed] [Google Scholar]
- 13.Levy AP. Application of pharmacogenomics in the prevention of diabetic cardiovascular disease: mechanistic basis and clinical evidence for utilization of the haptoglobin genotype in determining benefit from antioxidant therapy. Pharmacol Ther. 2006;112:501–512. doi: 10.1016/j.pharmthera.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Asleh R, Blum S, Kalet-Litman S, Alshiek J, Miller-Lotan R, Asaf R, Rock W, Aviram M, Milman U, Shapira C, Abassi Z, Levy AP. Correction of HDL dysfunction in individuals with diabetes and the haptoglobin 2-2 genotype. Diabetes. 2008;57:2794–2800. doi: 10.2337/db08-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cahill LE, Levy AP, Chiuve SE, Jensen MK, Wang H, Shara NM, Blum S, Howard BV, Pai JK, Mukamal KJ, Rexrode KM, Rimm EB. Haptoglobin genotype is a consistent marker of coronary heart disease risk among individuals with elevated glycosylated hemoglobin. J Am Coll Cardiol. 2013;61:728–737. doi: 10.1016/j.jacc.2012.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson M, Snell-Bergeon JK, Kinney GL, Lache O, Miller-Lotan R, Anbinder Y, Rewers MJ, Levy AP. Haptoglobin genotype predicts development of coronary artery calcification in a prospective cohort of patients with type 1 diabetes. Cardiovasc Diabetol. 2011;10:99. doi: 10.1186/1475-2840-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suleiman M, Aronson D, Asleh R, Kapeliovich MR, Roguin A, Meisel SR, Shochat M, Sulieman A, Reisner SA, Markiewicz W, Hammerman H, Lotan R, Levy NS, Levy AP. Haptoglobin polymorphism predicts 30-day mortality and heart failure in patients with diabetes and acute myocardial infarction. Diabetes. 2005;54:2802–2806. doi: 10.2337/diabetes.54.9.2802. [DOI] [PubMed] [Google Scholar]
- 18.Levy AP, Gerstein H, Lotan R, Ratner R, McQueen M, Lonn E, Pogue J. The effect of vitamin E supplementation on cardiovascular risk in diabetic individuals with different haptoglobin phenotypes. Diabetes Care. 2004;27:2767. doi: 10.2337/diacare.27.11.2767. [DOI] [PubMed] [Google Scholar]
- 19.Milman U, Blum S, Shapira C, Aronson D, Miller-Lotan R, Anbinder Y, Alshiek J, Bennett L, Kostenko M, Landau M, Keidar S, Levy Y, Khemlin A, Radan A, Levy AP. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2-2 genotype: a prospective double-blinded clinical trial. Arterioscler Thromb Vasc Biol. 2008;28:341–347. doi: 10.1161/ATVBAHA.107.153965. [DOI] [PubMed] [Google Scholar]
- 20.Blum S, Vardi M, Levy NS, Miller-Lotan R, Levy AP. The effect of vitamin E supplementation on cardiovascular risk in diabetic individuals with different haptoglobin phenotypes. Atherosclerosis. 2010;211:25–27. doi: 10.1016/j.atherosclerosis.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orchard TJ, Dorman JS, Maser RE, Becker DJ, Drash AL, Ellis D, LaPorte RE, Kuller LH. Prevalence of complications of IDDM by sex and duration: Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes. 1990;39:1116–1124. doi: 10.2337/diab.39.9.1116. [DOI] [PubMed] [Google Scholar]
- 22.Borhani NO, Kass EH, Langford HG, Payne GH, Remington RD, Stamler J. The hypertension detection and follow-up program. Prev Med. 1976;5:207–215. doi: 10.1016/0091-7435(76)90039-6. [DOI] [PubMed] [Google Scholar]
- 23.Warnick GR, Albers JJ. Heparin-Mn2+ quantitaion of high density lipoprotein cholesterol: an ultrafiltration procedure for lipemic samples. Clin Chem. 1978;24:900–904. [PubMed] [Google Scholar]
- 24.Allain CC, Poon LS, Chan CSG, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 25.Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19:476–482. [PubMed] [Google Scholar]
- 26.Ellis D, Buffone GJ. A new approach to the evaluation of proteinuric states. Clin Chem. 1977;23:666–670. [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koch W, Latz W, Eichinger M, Roguin A, Levy AP, Schömig A, Kastrati A. Genotyping of the common haptoglobin Hp 1/2 polymorphism based on PCR. Clin Chem. 2002;48:1377–1382. [PubMed] [Google Scholar]
- 29.Costacou T, Ferrell RE, Orchard TJ. Haptoglobin genotype: a determinant of cardiovascular complication risk in type 1 diabetes. Diabetes. 2008;57:1702–1706. doi: 10.2337/db08-0095. [DOI] [PubMed] [Google Scholar]
- 30.Levy NS, Vardi M, Blum S, Miller-Lotan R, Afinbinder Y, Cleary PA, Paterson AD, Bharaj B, Snell-Bergeon JK, Rewers MJ, Lache O, Levy AP. An enzyme linked immunosorbent assay (ELISA) for the determination of the human haptoglobin phenotype. Clin Chem Lab Med. 2013;51:1615–1622. doi: 10.1515/cclm-2013-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy AP. Haptoglobin genotype and vascular complications in patients with diabetes. New Engl J Med. 2000;343:969–970. doi: 10.1056/NEJM200009283431313. [DOI] [PubMed] [Google Scholar]
- 32.Levy AP, Hochberg I, Jablonski K, Resnick HE, Lee ET, Best L, Howard BV, Strong Heart Study Haptoglobin genotype is an independent risk factor for cardiovascular disease in individuals with diabetes: The Strong Heart Study. J Am Coll Cardiol. 2002;40:1984–1990. doi: 10.1016/s0735-1097(02)02534-2. [DOI] [PubMed] [Google Scholar]
- 33.Roguin A, Koch W, Kastrati A, Aronson D, Schomig A, Levy AP. Haptoglobin genotype is predictive of major adverse cardiac events in the 1-year period after percutaneous transluminal coronary angioplasty in individuals with diabetes. Diabetes Care. 2003;26:2628–2631. doi: 10.2337/diacare.26.9.2628. [DOI] [PubMed] [Google Scholar]
- 34.Costacou T, Ferrell RE, Ellis D, Orchard TJ. Haptoglobin genotype and renal function decline in type 1 diabetes. Diabetes. 2009;58:2904–2909. doi: 10.2337/db09-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orchard TJ, Sun W, Cleary P, Genuth S, Lachin JM, McGee P, Paterson AD, Raskin P, Anbinder Y, Levy AP, the DCCT/EDIC Research Group Haptoglobin Genotype and the Rate of Renal Function Decline in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study. Diabetes. doi: 10.2337/db13-0256. 10.2337/db13-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engström G, Hedblad B, Janzon L, Lindgärde F. Fatality of acute coronary events in relation to hypertension and low-grade inflammation: a population-based cohort study. J Hum Hypertens. 2006;20:581–586. doi: 10.1038/sj.jhh.1002037. [DOI] [PubMed] [Google Scholar]
- 37.Coelho C, Guerra A, Rego C, Breitenfeld L, Castro E, Rodrigues P, Laires MJ, Bicho M. Genetic polymorphisms of angiotensin-I converting enzyme, haptoglobin and angiotensinogen and oxidative stress parameters in 12 to 15-year-old adolescents. Rev Port Cardiol. 2006;25:677–690. [PubMed] [Google Scholar]
- 38.Delanghe JR, Duprez DA, De Buyzere ML, Bergez BM, Callens BY, Leroux-Roels GG, Clement DL. Haptoglobin polymorphism and complications in established essential arterial hypertension. J Hypertens. 1993;11:861–867. doi: 10.1097/00004872-199308000-00013. [DOI] [PubMed] [Google Scholar]
- 39.Delanghe JR, Duprez DA, De Buyzere ML, Bergez BM, Claeys LR, Leroux-Roels GG, Clement DL. Refractory hypertension is associated with the haptoglobin 2-2 phenotype. J Cardiovasc Risk. 1995;2:131–136. [PubMed] [Google Scholar]
- 40.Vardi M, Blum S, Levy AP. Haptoglobin genotype and cardiovascular outcomes in diabetes mellitus - natural history of the disease and the effect of vitamin E treatment. Meta-analysis of the medical literature. Eur J Intern Med. 2012;23:628–632. doi: 10.1016/j.ejim.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.