Abstract
A catalytic asymmetric Passerini reaction using tridentate indan (pybox) Cu(II) Lewis acid complex 4 with substrates capable of bidentate coordination has been achieved. The reaction occurs via ligand-accelerated catalysis.
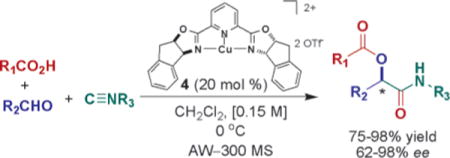
Strategies, reagents, and catalysts that enable systematic stereochemical and skeletal variation are central to effective diversity-oriented syntheses (DOS).1–4 The Passerini three–component coupling reaction (P-3CCR) (coupling of a carbonyl compound and an isocyanide with a carboxylic acid to form an α-acyloxycarboxamide) increases structural complexity; however, methods to control the stereochemical outcome of the carbon-carbon bond when simple achiral substrates are used are currently limited. Although several diastereoselective examples have been described that use chiral auxiliaries or chiral substrates to direct the α-addition of isocyanides,5 analogous enantioselective examples using chiral catalysts, which involve only one step, are less common.6,7 Herein, we report the development of a catalytic asymmetric P-3CCR giving rise to α-acyloxycarboxamides enantioselectively.
Passerini reactions performed in organic solvents are often sluggish and afford products in low yields unless either highly acidic carboxylic acids or unusually electrophilic carbonyl compounds are used. In contrast, electrophilic iminium ions, formed in situ from the corresponding four-component Ugi reactions undergo a more facile α-isocyanide addition. Since these findings underscore the importance of carbonyl activation we initially sought to use chiral Lewis acid chelation control with Cu(II)-derived Lewis acids to control the configurational outcome of the P-3CCR.8 Importantly, these conditions are sufficiently mild to be compatible with a one bead/one stock solution technology platform.9
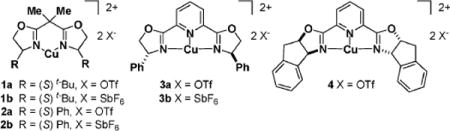
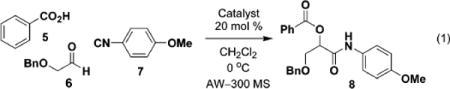
Our approach uses a Cu(II) complex and bidentate coordination with carbonyl substrates as a means to invoke stereocontrol of the newly formed C–C bond. Initial studies used bidentate (S,S)-bis(oxazolinyl) (box) (1 and 2)–Cu(II) and tridentate bis(oxazolinyl)pyridine (pybox) (3 and 4)–Cu(II) complexes.8,10 The reactions were performed in the presence of benzoic acid (5), (benzyloxy)acetaldehyde (6), and p-methoxyphenyl isocyanide (7) (Table 1). The ligand screen revealed that bispybox 4, derived from (1S,2R)-aminoindanol, was able to control the α-addition of the isocyanide with superior enantioselectivity and yield (entry 7, Table 1).
Table 1.
Optimization for the Lewis Acid Catalyzed P-3CCR
| |||||
|---|---|---|---|---|---|
| entrya | catalystb | time (h) (0 °C) | [conc] (M) | % yieldc | % eed |
| 1 | 1a | 18 | 0.25 | 71 | 50 |
| 2 | 1be | 18 | 0.25 | 63 | 56 |
| 3 | 2a | 14 | 0.50 | 93 | 64 |
| 4 | 2be | 8 | 0.50 | 87 | 63 |
| 5 | 3a | 18 | 0.25 | 90 | 85 |
| 6 | 3be | 18 | 0.25 | 86 | 79 |
| 7 | 4f | 18 | 0.15 | 93 | 97 |
| 8 | g | 36 (40 °C) | 0.15 | <5 | ND |
| 9 | h | 36 (40 °C) | 0.15 | <5 | ND |
6 was premixed with 20 mol % catalyst and cooled to 0 °C.
Homogeneous solution.
Isolated by column chromatography.
Determined by HPLC using a Chiralcel OD column.
AgCl was removed using a GHP Acrodisc 13 0.20 μm filter.
For optimal ee’s, the ligand was crystallized prior to use.
Cu(OTf)2 only.
Ligand of 4 only. ND, not determined.
The Cu(II) complex was stirred in methylene chloride until a homogeneous solution was obtained. When the SbF6− complexes 1b, 2b, and 3b were used, a homogeneous solution was difficult to achieve, even after filtration through a GHP Acrodisc 13 0.20 μm filter. (Benzyloxy)acetaldehyde (6) was then added to the solution, which was cooled to 0 °C. AW-300 MS (molecular sieves) were added to this solution followed by compounds 5 and 7, which were mixed together and added to the reaction mixture by a single syringe pump at a rate that ended in complete delivery after 4 h. The asymmetric induction was found to depend on the rate of the simultaneous addition of 5 and 7. The most promising results were achieved when a 4 h protocol was used (entries 1–7, Table 1).
Anhydrous conditions were necessary for high enantioselectivity. We attribute diminished ee’s to the increased rate of the background reaction as the P-3CCR, when conducted in the presence of water (no chiral Lewis acid), is known to experience a 20-fold rate enhancement.11 Water also leads to the hydrated form of the Cu(II) Lewis acid and decreases its ability to activate the carbonyl. The SbF6− counterion had a minimal effect on the overall enantioselectivity of the reaction (entries 2, 4, and 6, Table 1) as compared to instances in which the OTf- counterion was used (entries 1, 3, 5, and 4). It is interesting to note that when the reaction was performed only in the presence of the Cu(OTf)2 or solely in the presence of the ligand, there was little effect on the overall rate of reaction and enantioselectivity (entries 8 and 9, Table 1).
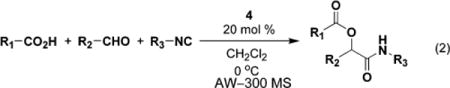
We next explored the substrate scope with different aliphatic and aromatic aldehydes and isocyanides. In all cases, we were able to obtain the desired product in fair to excellent yields and enantioselectivities (Table 2). (Benzyloxy)acetaldehyde (6) was the best carbonyl substrate used in the set as noted in entries 2, 4–7 (Table 2), giving yields ranging from 83 to 95% and ee’s from 72 to 98%. As noted in entries 3–16 (Table 2), benzoic acid was used predominantly as the acid component in the reaction; use of phenylacetic acid did not give high enantioselectivity (entries 1 and 2; Table 2), although the overall yields were comparable.
Table 2.
Generality of the Catalyzed P-3CCR Using Cu(II) Indan Pybox Catalyst
| ||||||
|---|---|---|---|---|---|---|
| entrya | R1 | R2 | R3 | product | % yieldb | % eec |
| 1 | PhCH2 (9) | 2-furyl (10) | PhCH2 (11) | 16 | 83 | 62 (R)d |
| 2 | PhCH2 (9) | BnOCH2 (6) | PhCH2 (11) | 17 | 87 | 72 |
| 3 | Ph (5) | 2-thiophenecarboxyl (12) | t-butyl (13) | 18 | 95 | 82 (R) |
| 4 | Ph (5) | BnOCH2 (6) | n-butyl (14) | 19 | 87 | 88 |
| 5 | Ph (5) | BnOCH2 (6) | n-pentyl (15) | 20 | 83 | 89 |
| 6 | Ph (5) | BnOCH2 (6) | PhCH2 (11) | 21 | 89 | 93 |
| 7 | Ph (5) | BnOCH2 (6) | t-butyl (13) | 22 | 95 | 98 |
| 8 | Ph (5) | 2-furyl (10) | p-MeOPh (7) | 23 | 98 | 91 (R) |
| 9 | Ph (5) | 2-furyl (10) | PhCH2 (11) | 24 | 90 | 75 (R) |
| 10 | Ph (5) | 2-furyl (10) | t-butyl (13) | 25 | 97 | 89 (R) |
| 11 | Ph (5) | 2-furyl (10) | n-butyl (14) | 26 | 82 | 78 (R) |
| 12 | Ph (5) | 2-furyl (10) | n-pentyl (15) | 27 | 82 | 78 (R) |
| 13 | Ph (5) | 2-thiophenecarboxyl (12) | p-MeOPh (7) | 28 | 95 | 89 (R) |
| 14 | Ph (5) | 2-thiophenecarboxyl (12) | PhCH2 (11) | 29 | 87 | 75 (R) |
| 15 | Ph (5) | 2-thiophenecarboxyl (12) | n-butyl (14) | 30 | 76 | 64 (R) |
| 16 | Ph (5) | 2-thiophenecarboxyl (12) | n-pentyl (15) | 31 | 75 | 60 (R) |
[0.15 M] final.
Isolated yield.
See the Supporting Information for HPLC conditions.
Inferred absolute stereochemistry based on X-ray crystal analysis with 5-bromo-2-furaldehyde as the substrate.
The choice of isocyanide also had an effect on both the rate of reaction and selectivity. tert-Butyl isocyanide (13) and p-methoxyphenyl isocyanide (7) afforded the highest yields and ee’s of products. In addition, the overall steric encumbrance of the isocyanides did not seem to have an effect on the enantioselectivity of the reaction. Aromatic isocyanides and aliphatic isocyanides lead to approximately equivalent ee’s.
In cases using carbonyl substrates that are capable of monodentate coordination only (e.g., benzaldehyde), the tridentate bis(oxazolinyl)pyridine (pybox)–Cu(II) complex (4) failed to direct the α-addition of the isocyanide with any significant selectivity. The catalytic asymmetric P-3CCR utilizing chiral Cu(II) Lewis acids appears, therefore, to be limited to bidentate coordinating substrates such as 6, 10, and 12.
In an attempt to harness the potential of this catalytic asymmetric P-3CCR, substrates were selected to create a complex molecular skeleton by generating a product-equals-substrate intermediate. Scheme 1 illustrates the use of components that give rise to a consecutive P-3CC/intramolecular Diels-Alder reaction producing a tricyclic lactone 33.
Scheme 1.
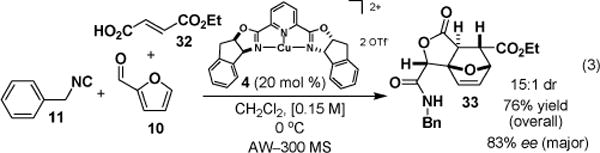
Complexity-Generating Reaction Forming Tricyclic Lactone 33
In summary, we report a catalytic asymmetric P-3CCR that uses tridentate bis(oxazolinyl)pyridine (pybox)–Cu(II) complex 4 and bidentate coordinating substrates 6, 10, and 12 to achieve products in up to 98% yield and 98% ee. The implications of this enantioselective pathway for DOS are now being investigated.
Supplementary Material
Acknowledgments
This research was supported by a grant from the NIGMS supporting the Harvard University CMLD. We thank Dr. Richard Staples for X-ray crystal structure analysis. P.R.A. acknowledges an NIH postdoctoral fellowship. C.C.L. acknowledges the Harvard College Research Program (HCRP) for a summer fellowship. S.L.S. is an Investigator at the Howard Hughes Medical Institute.
Footnotes
Supporting Information Available: General experimental procedures, characterization data, and an X-ray crystallographic file (CIF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Schreiber SL. Science. 2000;287:1964–1969. doi: 10.1126/science.287.5460.1964. [DOI] [PubMed] [Google Scholar]; (b) Schreiber SL. Chem Eng News. 2003;81:51–61. [Google Scholar]
- 2.Burke MD, Schreiber SL. Angew Chem Int Ed. 2004;43:46–58. doi: 10.1002/anie.200300626. [DOI] [PubMed] [Google Scholar]
- 3.(a) Burke MD, Berger EM, Schreiber SL. Science. 2003;302:613–616. doi: 10.1126/science.1089946. [DOI] [PubMed] [Google Scholar]; (b) Sello JK, Andreana PR, Lee D, Schreiber SL. Org Lett. 2003;5:4125–4127. doi: 10.1021/ol035773h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.For recent reviews:; (a) von Wangelin AJ, Neumann H, Goerdes D, Klaus S, Struebing D, Beller M. Chem Eur J. 2003;9:4286–4294. doi: 10.1002/chem.200305048. [DOI] [PubMed] [Google Scholar]; (b) Ugi L, Werner B, Doemling A. Molecules. 2003;8:53–66. [Google Scholar]; (c) Domling A. Curr Opin Chem Biol. 2002;6:306–313. doi: 10.1016/s1367-5931(02)00328-9. [DOI] [PubMed] [Google Scholar]; (d) Domling A, Ugi I. Angew Chem Int Ed. 2000;39:3168–3210. doi: 10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]; (e) Bienayme H, Hulme C, Oddon G, Schmitt P. Chem Eur J. 2000;6:3321–3329. doi: 10.1002/1521-3765(20000915)6:18<3321::aid-chem3321>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 5.Diastereoselective multicomponent coupling reactions:; (a) Cuny G, Gamez-Montano R, Zhu J. Tetrahedron. 2004;60:4879–4885. [Google Scholar]; (b) Frey R, Galbraith SF, Guelfi S, Lamberth C, Zeller M. Synlett. 2003:1536–1538. [Google Scholar]; (c) Ross GF, Herdtweck E, Ugi I. Tetrahedron. 2002;58:6127–6133. [Google Scholar]; (d) Oertel K, Zech G, Kunz H. Angew Chem Int Ed. 2000;39:1431–1433. doi: 10.1002/(sici)1521-3773(20000417)39:8<1431::aid-anie1431>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]; (e) Ziegler T, Kaisers H-J, Schlomer R, Koch C. Tetrahedron. 1999;55:8397–8408. [Google Scholar]; (f) Linderman RJ, Binet S, Petrich SR. J Org Chem. 1999;64:336–337. [Google Scholar]; (g) Bock H, Ugi I. J Prakt Chem. 1997;339:385–389. [Google Scholar]; (h) Semple JE, Wang PC, Lysenko Z, Joullie MM. J Am Chem Soc. 1980;102:7505–7510. [Google Scholar]; (i) Ugi I, Offermann K, Herlinger H, Marquarding D. Liebigs Ann Chem. 1967;709:1–10. doi: 10.1002/jlac.19677090102. [DOI] [PubMed] [Google Scholar]; (j) Ugi I, Offermann K. Angew Chem. 1963;75:917. [Google Scholar]
- 6.Kusebauch U, Beck B, Messer K, Herdtweck E, Domling A. Org Lett. 2003;5:4021–4024. doi: 10.1021/ol035010u. [DOI] [PubMed] [Google Scholar]
- 7.For the Passerini-type reaction giving rise to R-hydroxycarboxamide:; Denmark SE, Fan Y. J Am Chem Soc. 2003;125:7825–7827. doi: 10.1021/ja035410c. [DOI] [PubMed] [Google Scholar]
- 8.(a) Evans DA, Rovis T, Johnson JS. Pure Appl Chem. 1999;71:1407–1415. [Google Scholar]; (b) Evans DA, Kozlowski MC, Murry JA, Burgey CS, Connell B, Staples RJ. J Am Chem Soc. 1999;121:669–685. [Google Scholar]
- 9.Stavenger RA, Schreiber SL. Angew Chem Int Ed. 2001;40:3417–3421. doi: 10.1002/1521-3773(20010917)40:18<3417::aid-anie3417>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 10.For the synthesis of pybox ligand 4:; Davies IW, Gerena L, Lu N, Larsen RD, Reider PJ. J Am Chem Soc. 1996;61:9629–9630. [Google Scholar]
- 11.(a) Pirrung MC, Sarma KD. J Am Chem Soc. 2004;126:444–445. doi: 10.1021/ja038583a. [DOI] [PubMed] [Google Scholar]; (b) de Nooy AEJ, Masci G, Crescenzi V. Macromolecules. 1999;32:1318–1320. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


