Table 2.
Generality of the Catalyzed P-3CCR Using Cu(II) Indan Pybox Catalyst
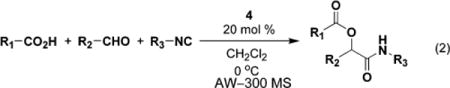
| ||||||
|---|---|---|---|---|---|---|
| entrya | R1 | R2 | R3 | product | % yieldb | % eec |
| 1 | PhCH2 (9) | 2-furyl (10) | PhCH2 (11) | 16 | 83 | 62 (R)d |
| 2 | PhCH2 (9) | BnOCH2 (6) | PhCH2 (11) | 17 | 87 | 72 |
| 3 | Ph (5) | 2-thiophenecarboxyl (12) | t-butyl (13) | 18 | 95 | 82 (R) |
| 4 | Ph (5) | BnOCH2 (6) | n-butyl (14) | 19 | 87 | 88 |
| 5 | Ph (5) | BnOCH2 (6) | n-pentyl (15) | 20 | 83 | 89 |
| 6 | Ph (5) | BnOCH2 (6) | PhCH2 (11) | 21 | 89 | 93 |
| 7 | Ph (5) | BnOCH2 (6) | t-butyl (13) | 22 | 95 | 98 |
| 8 | Ph (5) | 2-furyl (10) | p-MeOPh (7) | 23 | 98 | 91 (R) |
| 9 | Ph (5) | 2-furyl (10) | PhCH2 (11) | 24 | 90 | 75 (R) |
| 10 | Ph (5) | 2-furyl (10) | t-butyl (13) | 25 | 97 | 89 (R) |
| 11 | Ph (5) | 2-furyl (10) | n-butyl (14) | 26 | 82 | 78 (R) |
| 12 | Ph (5) | 2-furyl (10) | n-pentyl (15) | 27 | 82 | 78 (R) |
| 13 | Ph (5) | 2-thiophenecarboxyl (12) | p-MeOPh (7) | 28 | 95 | 89 (R) |
| 14 | Ph (5) | 2-thiophenecarboxyl (12) | PhCH2 (11) | 29 | 87 | 75 (R) |
| 15 | Ph (5) | 2-thiophenecarboxyl (12) | n-butyl (14) | 30 | 76 | 64 (R) |
| 16 | Ph (5) | 2-thiophenecarboxyl (12) | n-pentyl (15) | 31 | 75 | 60 (R) |
[0.15 M] final.
Isolated yield.
See the Supporting Information for HPLC conditions.
Inferred absolute stereochemistry based on X-ray crystal analysis with 5-bromo-2-furaldehyde as the substrate.
