Figure 2.
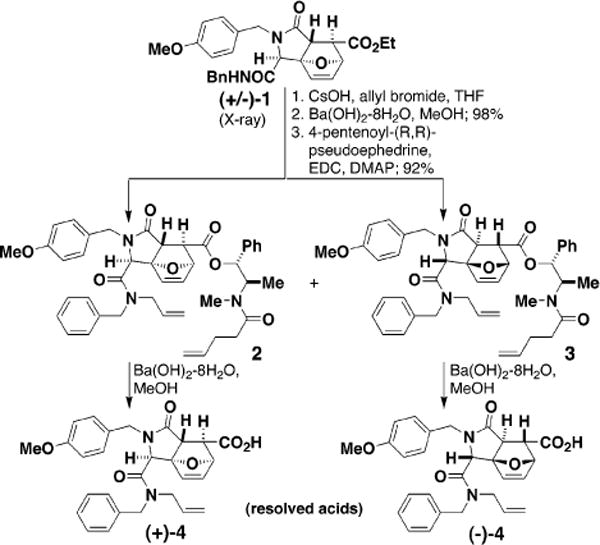
Racemic tricycle 1 was acylated with 4-pentenoyl-(R,R)-pseudoephedrine, yielding diastereomers that were purified using silica gel chromatography. Following saponification, the resolved acids (+)-4 and (−)-4 were used in subsequent experiments.
