Figure 1. The ATPase activity of SecA is reduced in the E-to-Q mutants.
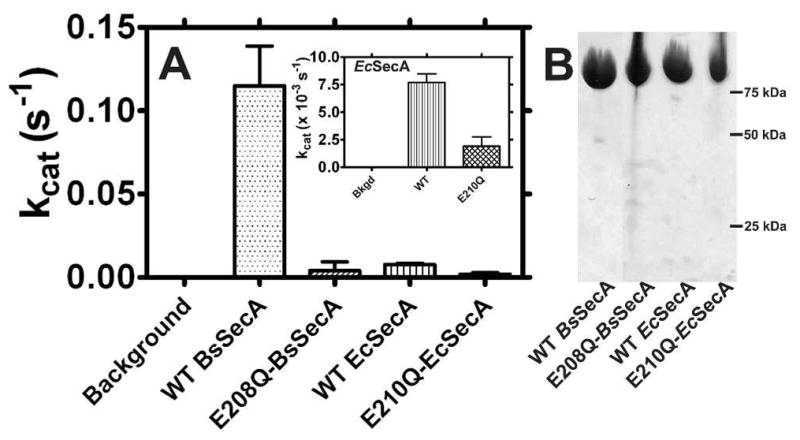
(A) ATP hydrolysis rates of WT and mutant SecAs were measured using the Malachite Green phosphate-release assay. Reactions at 25 °C containing 1–2 μM SecA and 2 mM Mg-ATP in KEMT buffer were monitored at 10–15 minute intervals for 30–90 minutes. The observed activity of E210Q-EcSecA is so low that it could be attributable to contaminating enzymes despite the high protein purity (see text). (B) Coomasie-blue stained SDS-PAGE gel of purified SecA variants.
