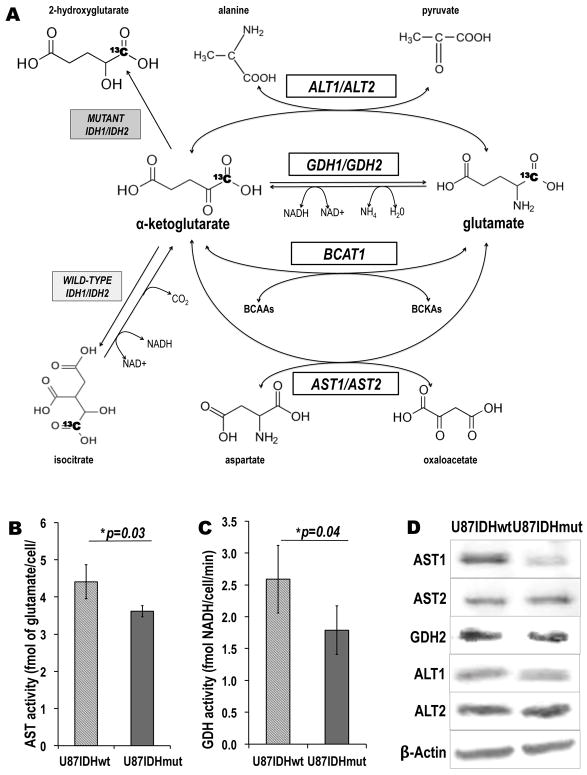
Figure 4. In addition to BCAT1, the presence of IDH1 mutation is associated with decreased activities and protein levels of additional enzymes catalyzing α-KG-to-glutamate conversion.
(A) Schematic of [1-13C] α-KG metabolism illustrating four enzymatic pathways through which α-KG can be metabolized to glutamate: BCAT1 (branched chain aminotransferase 1; BCAAs: branched-chain aminoacids; BCKAs: branched-chain ketoacids), AST1 and AST2 (aspartate aminotransferase 1 and 2), GDH1 and GDH2 (glutamate dehydrogenase 1 and 2) and ALT1 and ALT2 (Alanine Aminotransferase 1 and 2). The reactions catalyzed by wild-type and mutant IDH1 and IDH2 are also shown (For all enzymes: 1=cytoplasmic isoform; 2=mitochondrial isoform). The 13C label at the C1 position of α-KG is highlighted in bold. (B) AST and (C) GDH enzymatic activities as measured by spectrophotometric assays. The activities of both enzymes are significantly decreased in mutant IDH1 cells as compared to wild-type cells (*p<0.05; **p<0.01; n=4 per cell line and per enzyme). (D) Western blots for the AST1, AST2, GDH2, ALT1 and ALT2 enzymes. BCAT1 and AST1 protein levels were significantly decreased in U87IDHmut cells, whereas the rest of the enzymes levels were unchanged. β-Actin was used as a loading control. (Note: GDH1 was below detection level).