Abstract
Prior analyses of the Cooperative Study of Sickle Cell Disease (CSSCD) newborn cohort identified elevated white blood cell (WBC) count, low baseline hemoglobin and dactylitis between the age 1 and 2 years as markers of severe disease. Reticulocytosis was also associated with severe disease. Here we further analyzed data collected on enrolled CSSCD infants for the predictive value of those markers for stroke and death later in life. Three hundred fifty-four CSSCD subjects were identified who had absolute reticulocyte counts (ARC) measured during infancy (2 to 6 months of age). Infants with higher ARC had significantly increased risk of stroke or death during childhood; lower hemoglobin levels also increased the risk, but to a lesser degree than ARC. WBC levels and dactylitis were not predictive of death or stroke. These data suggest that reticulocytosis among asymptomatic infants with sickle cell anemia is associated with an increased risk of death or stroke during childhood.
Keywords: sickle cell anemia, reticulocytosis
Introduction
The clinical phenotype of sickle cell anemia (HbSS, SCA) is highly variable and currently impossible to predict before clinical symptoms begin. Recently, in a single-center pilot study, steady state absolute reticulocyte count (ARC), but not hemoglobin or white blood cell (WBC) count, during early infancy was associated with an increased rate of early hospital admission for painful crisis, acute chest syndrome and splenic sequestration. [1] Infants with increased levels of reticulocytosis were hospitalized at an earlier age and had a four-fold higher cumulative frequency of hospitalizations during the first three years of life. None of the enrolled infants in the study died or had a stroke, arguably the two most severe complications of SCA. [1] In a separate study, reticulocytosis at the time of Transcranial Doppler (TCD) screening (median age 6.2 years, range 2.0–11.2 years) was correlated with cerebrovascular disease in a cohort of Brazilian SCA patients. [2]
Steady state reticulocytosis during the second year of life was previously associated with more severe SCA in analyses of the newborn cohort included in the Cooperative Study of Sickle Cell Disease (CSSCD). [3] Low baseline hemoglobin (<7 g/dL), steady state leukocytosis, and dactylitis were also associated with severe SCA. The reticulocyte percentage did not significantly affect the multi-variate model that included WBC count, hemoglobin and dactylitis. Analysis of the Dallas Newborn Cohort in 2008, found that none of the previously identified disease severity predictors, either in combination or alone, reliably identified those pediatric SCA patients who had adverse events. [4] Thus, additional disease severity predictors are being sought in order to guide treatment decisions in young children with SCA. The hypothesis tested here is that very early detection (during infancy), of reticulocytosis, anemia, or leukocytosis is predictive of later major adverse events (i.e. stroke and death) in SCA. For this purpose the CSSCD data were re-analyzed.
Methods
De-identified data from the CSSCD was obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center following IRB approval from Children’s National Medical Center and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). For this study, we included infants whose first CSSCD visit was within 196 days of birth and we used steady state hematologic data from CSSCD study visits during early infancy, defined as 2–6 months of age (60–196 days). The association of infant reticulocytosis with painful crisis, acute chest syndrome and other SCA-related complications were studied previously using a modern cohort of infants with sickle cell disease. [1] However, the two most severe outcomes of SCA, stroke and death, were not present in that cohort. Therefore, archived data from the CSSCD cohort was analyzed to determine if early ARC could identify those pediatric SCA patients who are at the highest risk of stroke or death. An analysis data set was created using laboratory and event data (stroke or death) and duration of follow-up. For subjects who had more than one ARC recorded, the highest value was utilized. According to the CBC associated with the highest ARC, patients were divided into quartiles based on hemoglobin, WBC count and peak ARC. Kaplan-Meier estimates of event rates were calculated and compared among the four groups using the log-rank test. Cox regression analysis was used to adjust for other predictors. A value of p<0.05 was considered statistically significant. Data are presented as mean ± standard deviation (SD) unless otherwise indicated.
Results
The CSSCD data set included 440 infants with a diagnosis of HbSS who had their first visit within 196 days of age, 354 of these had at least one ARC measurement between the ages of 2 and 6 months. The mean age at entry of these 354 infants was 145 ± 33 days (range 64–196 days), 51% were male, the mean ARC value was 223 ± 154 K/uL, the mean Hb was 9.3 ± 1.4 g/dL, the mean WBC count was 10.6±3.9 K/uL. These subjects were followed for a median of 10.8 years (range 0.5 to 19.8 years). Thirteen infants (3.7%) had dactylitis before the age of six months. Demographic and hematologic data for each ARC quartile are shown in Table 1 and are consistent with previous reports. [1,5]
Table 1.
Demographic and Hematologic Data for each Absolute Reticulocyte Quartile.
| ARC Group (n) | M:F | Mean (SD) Age @ Time of ARC (days) | Mean (SD) ARC | Mean (SD) Hb | Mean (SD)WBC | # of death or stroke (%) |
|---|---|---|---|---|---|---|
| 4–104 (88) | 45:43 | 138 (35) | 68 (25) | 9.7 (1.4) | 9.9 (3.0) | 3 (3.4) |
| 105–193 (89) | 46:43 | 143 (33) | 144 (26) | 9.6 (1.4) | 10.2 (3.2) | 4 (4.5) |
| 194–307 (88) | 45:43 | 145 (32) | 237 (32) | 9.2 (1.2) | 10.2 (3.1) | 13 (14.8) |
| 308–863 (89) | 45:44 | 155 (30) | 443 (113) | 8.6 (1.4) | 12.2 (5.2) | 18 (20.2) |
| Correlation coefficient with ARC | N/A | 0.15 (p=0.0037) | 1.0 | −0.31 (p<0.0001) | 0.22 (p<0.0001) | N/A |
ARC=Absolute Reticulocyte Count, n=number in group, SD=standard deviation, Hb= hemoglobin, WBC=white blood cell count, N/A=not applicable
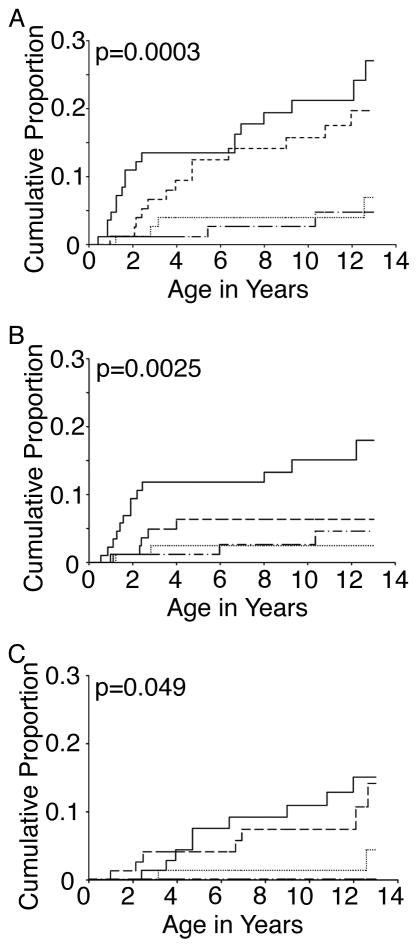
Of the 354 subjects, 38 had a stroke or died [18 subjects had been diagnosed with a stroke and 23 subjects died (including 3 of 18 subjects within the stroke group who later died)]. Mean age at time of stroke was 6.5±4.1 years (range 1.0 – 12.6 years), while the average age at the time of death was 3.4±3.4 years (range 0.5–12.2 years). Figure 1A shows the Kaplan-Meier plots of age at the time of stroke or death in the four groups that were divided into quartiles according to ARC from three to six months of age. Among the group with ARC ≥ 308, the most abrupt rise in death occurred before two years of age. The event rates for stroke or death at 13 years were 4.7% for ARC <105, 6.8% for ARC 105–193, 19.4% for ARC 194–307, and 26.7% for ARC ≥308, p=0.0003 by the log-rank test (Figure 1A). Thirteen (56.6%) of the non-stroke related deaths were attributed to infection; five of the patients who succumbed to infection were receiving penicillin prophylaxis.
Figure 1. Kaplan–Meier Analysis of time to death or stroke in the CSSCD stratified according to ARC.
Log-rank test was performed (p value shown in each panel) to compare the cumulative proportion of death and stroke (A), death (B), or stroke (C). Utilizing the steady state CBC associated with the highest ARC between 2 and 6 months of age, patients were divided into four groups by ARC [ARC 4–104 K/uL (dash-dot line), ARC 105–193 K/uL (dotted line), ARC 194–307 K/uL (dashed line), and ARC 308–863 K/uL (solid line)]
Because steady state hemoglobin and WBC counts were identified as important markers of disease severity in earlier reports, [3] the association of hemoglobin and WBC count early in life with death and stroke was also examined. Similar to the ARC, subjects were divided into quartiles based on steady state hemoglobin at the time of the highest ARC and Kaplan-Meier plots of time to stroke or death in the four groups were constructed (Supplementary Figure 1A). Those subjects with the lowest hemoglobin had the highest rate of stroke or death (24.7%) and those subjects with the highest steady state hemoglobin (>10.2 g/dL) had the lowest event rate (7.3%, p=0.016 by the log-rank test comparing all 4 groups). The event rate across the four WBC count groups did not differ significantly (Supplementary Figure 1D, p=0.16 by the log rank test). In multivariate Cox regression analysis, only ARC was independently associated with stroke or death (ARC p=0.011, hemoglobin p=0.21, WBC, p=0.45). Thirteen (3.7%) infants had dactylitis before 196 days of age. Although 3/13 (23.1%) died or had a stroke, dactylitis was not predictive for death or stroke either in univariate analysis (p=0.24) or when adjusting for ARC (data not shown, p=0.42 using Cox regression analysis).
Discussion
Review of archived CSSCD data demonstrated that anemia and reticulocytosis during early infancy were associated with a significant increase in stroke or death during childhood in support of the recent findings that increased reticulocytosis correlates with more SCA-associated hospitalizations. [1] Consistent with a previous report from the BABY HUG study, [6] those infants in the lowest quartile for hemoglobin had the highest event rate of the four hemoglobin groups, while there was no statistical difference in event rates between the four groups divided by steady state WBC count. The rate of stroke and death increased in a step wise fashion with increasing ARC, but there was not a consistent decrease in the number of events as the hemoglobin increased which would limit the utility of using hemoglobin as a risk stratification marker in young infants. Since leukocytosis in SCA patients is not usually manifest until after 6 months of age, [7–9] the lack of an association between leukocytosis and stroke or death was not predicted or identified in this cohort.
The down-regulation and silencing of HbF expression during human infancy represent a fundamental aspect of human ontogeny that ultimately causes the phenotypic manifestation of SCA. [10] As HbF expression decreases, HbS expression increases. The polymerization of sickle hemoglobin causes hemolysis and vaso-occlusion with resulting anemia and tissue hypoxia. In response, vigorous erythropoiesis with reticulocytosis becomes a lifelong feature of the disease. Prior studies demonstrated that the reticulocytes released into the circulation express plasma membrane adhesion markers, which may play an important role in SCA-related vasculopathy and organ damage. [11,12] Our analyses further support the notion that the magnitude of reticulocytosis during infancy may serve as a marker of the early hemolysis or subsequent disease severity. However, determination of the potential role that reticulocytes may play in SCA-related organ damage was not addressed in this study.
CSSCD data has been utilized for decades to study the manifestations of pediatric sickle cell disease in the US. Analyses of the data have varied according to differences in age, exclusion, and length of follow-up criteria. [3,13,14] Our analyses relied solely upon the archives of CSSCD data that were collected over a span of nearly 20 years. During that time, automated reticulocyte counting became available [15] and replaced manual counting. Therefore, our analyses include manual reticulocyte quantitation with noted inter-reporter variability [8] as well as later reticulocyte quantitation methods that included reference hematologic ranges for pediatric SCA patients from birth to 5 years of age [7]. When interpreting the mortality data in the CSSCD cohort, it is also important to consider that the cohort did not receive the nearly universal level of supportive and preventive care that is provided today in the United States and Europe (i.e. prophylactic penicillin, vaccination and TCD screening). Today, 95% of infants born with SCA will survive until their 18th birthday, due in large part to early identification of affected infants by newborn screening programs, early institution of penicillin prophylaxis and improved vaccinations with protein conjugate vaccines against streptococcus pneumoniae and haemophilus influenza type B. [16]
Overall, these data support the use of ARC during early infancy as a marker for SCA infants who are at highest risk for serious disease complications. These historic data provide insights into potential disease severity markers that may be useful in treatment decisions today or in the design of related studies. High levels of reticulocytosis during infancy may indicate a poor prognosis, and thus support early consideration of disease modifying therapies. Prospective studies are needed to determine if such an approach is warranted.
Supplementary Material
Steady state CBC associated with the highest ARC between 2 and 6 months of age was utilized to divide patients into four groups by Hb (A–C) [Hb 6.1–8.2 g/dL (dash-dot line), Hb 8.3–9.2 g/dL (dotted line), Hb 9.3–10.1 g/dL (dashed line), and Hb 10.2–15.4 g/dL (solid line)] or by WBC (D–F) [WBC 4,500–8,100/uL (dotted line), WBC 8,200–9,700/uL (dash-dot line), WBC 9,800–12,200/uL (dashed line), and 12,300–36,700/uL (solid line)]. Log-rank test was performed to compare the cumulative proportion of death and stroke (A and D), death (B and E), or stroke (C and F).
Key points.
Reticulocytosis among asymptomatic infants enrolled in the CSSCD is associated with an increased risk of death or stroke during childhood.
Acknowledgments
The authors thank Dr. Scott T. Miller for his reading and helpful comments for this manuscript. The Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases supported this work. This manuscript was prepared using Cooperative Study of Sickle Cell Disease (CSSCD) research materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center and do not necessarily reflect the opinions or views of the CSSCD or the NHLBI. Dr. Meier is also supported by Award Numbers UL1TR000075 and KL2TR000076 from the NIH National Center for Advancing Translational Sciences.
Footnotes
Authorship Contributions ERM designed the study, analyzed the data, and wrote the manuscript; ECW analyzed the data and edited the manuscript; JLM designed the study and wrote the manuscript.
Disclosure of Conflicts of Interest The authors have no conflicts of interest to disclose.
Financial Assistance/Conflicts of Interest: The Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases supported this work. Dr. Meier is supported by Award Numbers UL1TR000075 and KL2TR000076 from the NIH National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. The authors have no conflicts of interest to disclose.
References
- 1.Meier ER, Byrnes C, Lee YT, et al. Increased reticulocytosis during infancy is associated with increased hospitalizations in sickle cell anemia patients during the first three years of life. PLoS One. 2013;8:e70794. doi: 10.1371/journal.pone.0070794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silva CM, Giovani P, Viana MB. High reticulocyte count is an independent risk factor for cerebrovascular disease in children with sickle cell anemia. Pediatr Blood Cancer. 2011;56:116–121. doi: 10.1002/pbc.22680. [DOI] [PubMed] [Google Scholar]
- 3.Miller ST, Sleeper LA, Pegelow CH, et al. Prediction of adverse outcomes in children with sickle cell disease. New Engl J Med. 2000;342:83–89. doi: 10.1056/NEJM200001133420203. [DOI] [PubMed] [Google Scholar]
- 4.Quinn CT, Lee NJ, Shull EP, et al. Prediction of adverse outcomes in children with sickle cell anemia: a study of the Dallas Newborn Cohort. Blood. 2008;111:544–548. doi: 10.1182/blood-2007-07-100719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes RJ, Beckford M, Grandison Y, et al. The haematology of steady state homozygous sickle cell disease: frequency distributions, variation with age and sex, longitudinal observations. Br J Haematol. 1985;59:369–382. doi: 10.1111/j.1365-2141.1985.tb03002.x. [DOI] [PubMed] [Google Scholar]
- 6.Lebensburger JD, Miller ST, Howard TH, et al. Influence of severity of anemia on clinical findings in infants with sickle cell anemia: analyses from the BABY HUG study. Pediatr Blood Cancer. 2012;59:675–678. doi: 10.1002/pbc.24037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown AK, Sleeper LA, Miller ST, et al. Reference values and hematologic changes from birth to 5 years in patients with sickle cell disease. Cooperative Study of Sickle Cell Disease. Arch Pediatr Adolesc Med. 1994;148:796–804. doi: 10.1001/archpedi.1994.02170080026005. [DOI] [PubMed] [Google Scholar]
- 8.West MS, Wethers D, Smith J, Steinberg M. Laboratory profile of sickle cell disease: a cross-sectional analysis. The Cooperative Study of Sickle Cell Disease. J Clin Epidemiol. 1992;45:893–909. doi: 10.1016/0895-4356(92)90073-v. [DOI] [PubMed] [Google Scholar]
- 9.Nathan DG, Orkin SH, Ginsburg D, Look AT, editors. Nathan and Oski’s Hematology of Infancy and Childhood. Philadelphia: W.B. Saunders Company; 2003. p. 1864. [Google Scholar]
- 10.Manca L, Masala B. Disorders of the synthesis of human fetal hemoglobin. IUBMB Life. 2008;60:94–111. doi: 10.1002/iub.4. [DOI] [PubMed] [Google Scholar]
- 11.Styles LA, Lubin B, Vichinsky E, et al. Decrease of very late activation antigen-4 and CD36 on reticulocytes in sickle cell patients treated with hydroxyurea. Blood. 1997;89:2554–2559. [PubMed] [Google Scholar]
- 12.Setty BN, Kulkarni S, Stuart MJ. Role of erythrocyte phosphatidylserine in sickle red cell-endotheial adhesion. Blood. 2002;99:1564–1571. doi: 10.1182/blood.v99.5.1564. [DOI] [PubMed] [Google Scholar]
- 13.Bray GL, Muenz L, Makris L, Lessen LS. Assessing clinical severity in children with sickle cell disease. Preliminary results from a cooperative study. Am J Pediatr Hematol Oncol. 1994;16:50–54. [PubMed] [Google Scholar]
- 14.Gill FM, Sleeper LA, Weiner SJ, et al. Clinical events in the first decade in a cohort of infants with sickle cell disease. Cooperative Study of Sickle Cell Disease. Blood. 1995;86:776–783. [PubMed] [Google Scholar]
- 15.Tichelli A, Gratwohl A, Driessen A, et al. Evaluation of the Sysmex R-1000. An automated reticulocyte analyzer. Am J Clin Pathol. 1990;93:70–78. doi: 10.1093/ajcp/93.1.70. [DOI] [PubMed] [Google Scholar]
- 16.Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood. 2010;115:3447–3452. doi: 10.1182/blood-2009-07-233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Steady state CBC associated with the highest ARC between 2 and 6 months of age was utilized to divide patients into four groups by Hb (A–C) [Hb 6.1–8.2 g/dL (dash-dot line), Hb 8.3–9.2 g/dL (dotted line), Hb 9.3–10.1 g/dL (dashed line), and Hb 10.2–15.4 g/dL (solid line)] or by WBC (D–F) [WBC 4,500–8,100/uL (dotted line), WBC 8,200–9,700/uL (dash-dot line), WBC 9,800–12,200/uL (dashed line), and 12,300–36,700/uL (solid line)]. Log-rank test was performed to compare the cumulative proportion of death and stroke (A and D), death (B and E), or stroke (C and F).