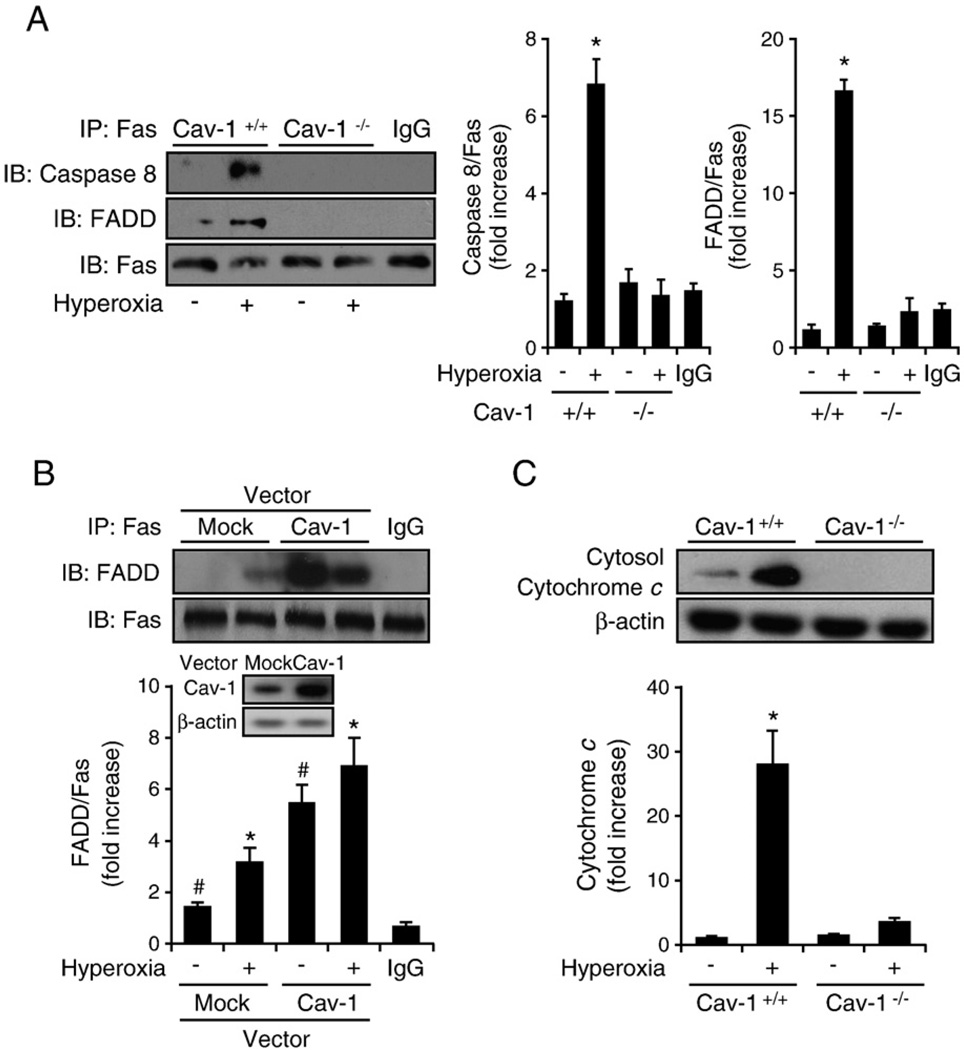
Fig. 2.
Cav-1 facilitated the hyperoxia-induced Fas–FADD/caspase 8 interaction in the absence of the Fas ligand. Primary mouse lung epithelial cells and Beas-2B human epithelial cells were used in these experiments. Cells were exposed to room air (i.e., 20.8% oxygen + 78.08% nitrogen) or hyperoxia (95% oxygen + 5% balanced nitrogen) conditions. At designated times (i.e., after 4, 24, or 48 h), the cells were collected and subjected to co-IP assay or Western blot analysis. DISC formation was determined by detecting the interaction of Fas–FADD/caspase 8. (A) The deletion of Cav-1 disrupted hyperoxia-induced DISC formation. Primary mouse lung epithelial cells from either wild-type C57BL/6 mice (Cav-1+/+) or Cav-1−/− mice were exposed to room air or hyperoxia (4 h) conditions. Co-IP assays between Fas and FADD and Fas and caspase 8 were performed as described previously. (B) Overexpressing Cav-1 facilitated Fas–FADD interaction after hyperoxia. Beas-2B cells were used in these experiments and infected with adeno-lacZ (control) and adeno-Cav-1, as described previously [41]. Cells were then exposed to hyperoxia (4 h), and co-IP assays were performed between Fas and FADD. (C) Deletion of Cav-1 prevented the release of cytochrome c after hyperoxia. Primary mouse lung epithelial cells from either wild-type C57BL/6 mice or Cav-1−/− mice were exposed to hyperoxia. After 24 h, the level of cytosol cytochrome c was determined by Western blot analysis, as described previously [28]. All experiments presented were repeated using three independent assays with similar results. *P<0.05,#P<0.01.