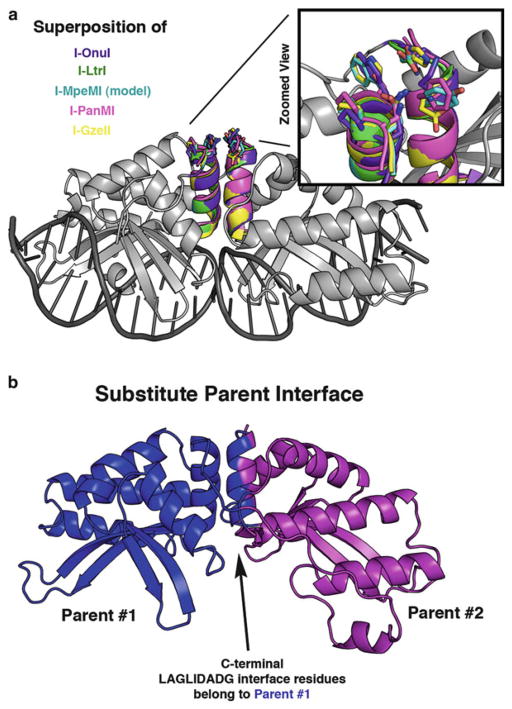
Fig. 8.
Structural representation of areas for optimization of partially active chimeras. (a) The I-OnuI structure (gray) is shown with superimposed LAGLIDADG helices from five different enzymes: I-OnuI helices are colored purple, I-LtrI are green, I-MpeMI (homology model) are cyan, I-PanMI are magenta, and I-GzeII are yellow. Side chains are depicted as “sticks” at the DNA-distal end of the LAGLIDADG helices. A magnified view highlights the lack of conservation at these positions. (b) A significant portion of the chimeric interface is formed between the two adjacent LAGLIDADG helices. Residues from a single parent enzyme (blue) can be substituted onto BOTH sides of the LAGLIDADG interface to help overcome problems with an otherwise incompatible chimeric interface