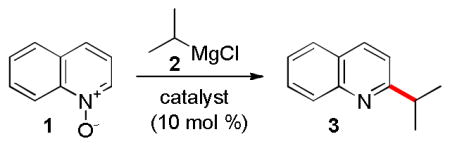
Table 1.
Optimization of the Reaction Conditions.a
| |||||
|---|---|---|---|---|---|
| entry | catalyst | additiveb | solvent | temp (°C) | yield % |
| 1 | – | – | Et2O | 23 | 0 |
| 2 | CuCl | – | Et2O | 23 | 53 |
| 3 | FeCl2 | – | Et2O | 23 | 36 |
| 4c | FeCl2/SIMes | – | THF | 23 | 0 |
| 5 | CoCl2 | – | Et2O | 23 | 6 |
| 6 | Ni(PPh3)2Cl2 | – | THF | 23 | 3 |
| 7 | NiCl2·dme | – | Et2O | 23 | 0 |
| 8 | CuCl2 | – | Et2O | 23 | 17 |
| 9 | CuBr | – | Et2O | 23 | 21 |
| 10 | CuCl | – | THF | 23 | 45 |
| 11 | CuCl | – | PhMe | 23 | 38 |
| 12 | CuCl | – | Et2O | 0 | 44 |
| 13 | CuCl | – | Et2O | −20 | 25 |
| 14 | CuCl | – | Et2O | 50 | 42 |
| 15 | CuCl | LiCl | Et2O | 23 | 67 |
| 16 | CuCl | ZnBr2 | Et2O | 23 | 0 |
| 17 | CuCl | tmeda | Et2O | 23 | 2 |
| 18 | CuCl | MgCl2 | Et2O | 23 | 95 |
| 19 | CuCl | MgF2 | Et2O | 23 | 37 |
| 20 | CuCl | LiF | Et2O | 23 | 96 |
Reaction conditions: 1 (0.17 mmol), 2 (1.5 equiv), catalyst (10 mol %), in the solvent (1 mL) under Ar for 12 h.
1.5 equiv
SIMes = 1,3-bis(2,4,6-trimethylphenyl)imidazol-2-ylidene.
