Table 1.
Optimization of the reaction conditions.a
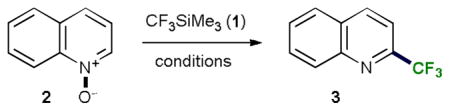
| |||||
|---|---|---|---|---|---|
| Entry | Base/fluoride (equiv) | Solvent | T (°C) | 2 : 3 : quinolineRatiob | Yieldb of 3, % |
| 1 | TBAF (0.2) | THF | 23 | 7 : 1 : 1 | 11 |
| 2 | TBAF (0.2) | PhCH3/THF (1:1) | 23 | 1.7 : 1.5 : 1 | 34 |
| 3 | TBAF (0.2) | PhCH3/THF (1:1) | 65 | 10 : 1 : 1.5 | 8 |
| 4 | TASF (1.1) | PhCH3/THF (1:1) | 23 | 2 : 2.5 : 1 | 44 |
| 5 | CsF (1.1) | PhCH3/THF (1:1) | 23 | 1.6 : 1.7 : 1 | 34 |
| 6c | TASF (1.1) | PhCH3/THF (1:1) | 23 | 1.3 : 2.5 : 1 | 50 |
| 7d | TASF (1.1) | THF | 23 | 1.5 : 2.1 : 1 | 44 |
| 8 | KOt-Bu (1.1) | THF | 23 | 1 : 6.8 : 17.3 | 26 |
| 9 | KOt-Bu (3) | THF | 23 | 1 : 3.2 : 5.8 | 32 |
| 10e | KOt-Bu (3) | THF | −20 | 1 : 29 : 3.2 | 87 |
Reaction conditions: 2 (0.35 or 1 mmol), 1 (2.5 equiv), in the solvent (c=0.7 M) under Ar for 2 h.
Determined by 1H NMR.
(CuOTf)2·PhH (5 mol %) was added.
MgCl2 (2 equiv) was added.
c=0.2 M.
