Figure 3. uPAR-deficient mice exhibit impaired clearance of apoptotic cells.
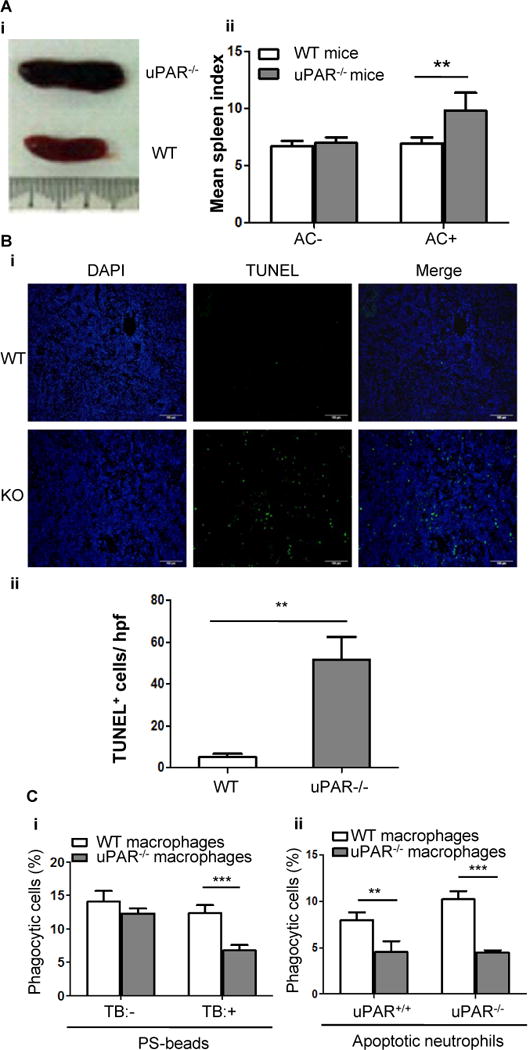
(A) Apoptotic cells (2×107 per mouse) were intravenously injected into WT and uPAR−/− mice (n=7) every day for 7 days. Shown is the representative gross appearance of spleens on day 8 (i). (ii) The mean spleen index of WT and uPAR−/− mice that received injection of PBS (AC−) and apoptotic cells (AC+) is shown.**, p<0.01. (B) Spleens of the above experiments were further processed for paraffin-embedded sections. Apoptotic cells were detected by TUNEL method. Nuclei were counterstained with DAPI. Representative images (20×) were shown (i, bars =100 μm). (ii) TUNEL-positive cells were enumerated in 10 randomly chosen high-power fields (hpf). **, p<0.01. (C) Internalization of PS-coated beads or apoptotic neutrophils in vivo. As described in the Materials and Methods, 4×107 NBD-PC-labeled PS-beads (i) or apoptotic neutrophils isolated from WT or uPAR−/− mice (ii) were intravenously injected into WT and uPAR−/− mice (n=5). Six hours after injection, spleens were harvested and homogenized, and F4/80-positive macrophages were purified. After treatment with or without TB, internalization of PS-coated beads was analyzed by flow cytometry (i). After treatment with TB, internalization of uPAR+/+ and uPAR−/− apoptotic neutrophils was analyzed by flow cytometry (ii). **, p<0.01, ***, p<0.001.
