Figure 7. Integrity of lipid rafts and Rac1 activation are required for uPAR mediated-internalization of apoptotic cells.
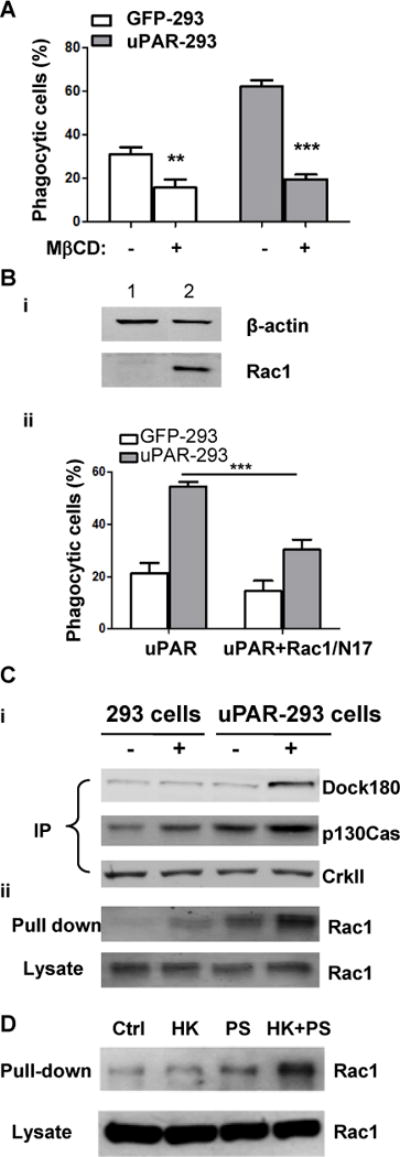
(A) After preincubation with or without 2.5 mM mβCD for 30 minutes, apoptotic cells were co-cultured with EGFP-293 or uPAR-293 cells. The internalization of apoptotic cells was analyzed by flow cytometry as described in the legend for Figure 4. ***, p< 0.001. (B) EGFP-293 or uPAR-293 cells were transfected with empty vector (lane 1) or Rac1/N17 (lane 2) cDNA for 48 hours, the overexpression of Rac1/N17 was detected by Western blotting using anti-Rac1 antibody with β-actin serving as a loading control (i). Internalization of apoptotic cells was analyzed (ii). **, p<0.01. (C) EGFP-293 cells and uPAR-293 cells were starved in DMEM plus 2% FBS for 8 hours, and incubated without (lanes 1 and 3) or with (lane 2 and 4) 1.0 μM PS liposome plus 600 nM HK for 1 hour in basal DMEM containing 0.35% BSA and 50 μM ZnCl2. Cell lysates were subjected to immunoprecipitation with anti-CrkII antibody (A) and Rac1 activity assay (B), respectively. The immunoprecipitates using anti-CrkII antibody were analyzed by immunoblotting with MoAb against p130Cas and polyclonal antibodies against CrkII and Dock180. Active GTP-loaded form of Rac1 and total Rac1 in cell lysates were detected by immunoblotting. (D) After starvation in DMEM plus 2% FBS for 8 hours, uPAR-293 cells were incubated with PBS (control, Ctrl), 600 nM HK (HK), 1.0 μM PS liposome (PS), or 600 nM HK plus 1.0 μM PS liposome (HK+PS) for 1 hour in DMEM containing 0.35% BSA and 50 μM ZnCl2. Cell lysates were incubated with GST-PAK1 conjugated with Glutathione Sepharose 4B beads and active GTP-loaded form of Rac1 (Pull-down) and total Rac1 in cell lysates (Lysate) were detected by immunoblotting.
