Figure 1. Oligonucleotides with biotin labels on the 3′-end or 5′-end have revealed directional biased activity of helicases on DNA.
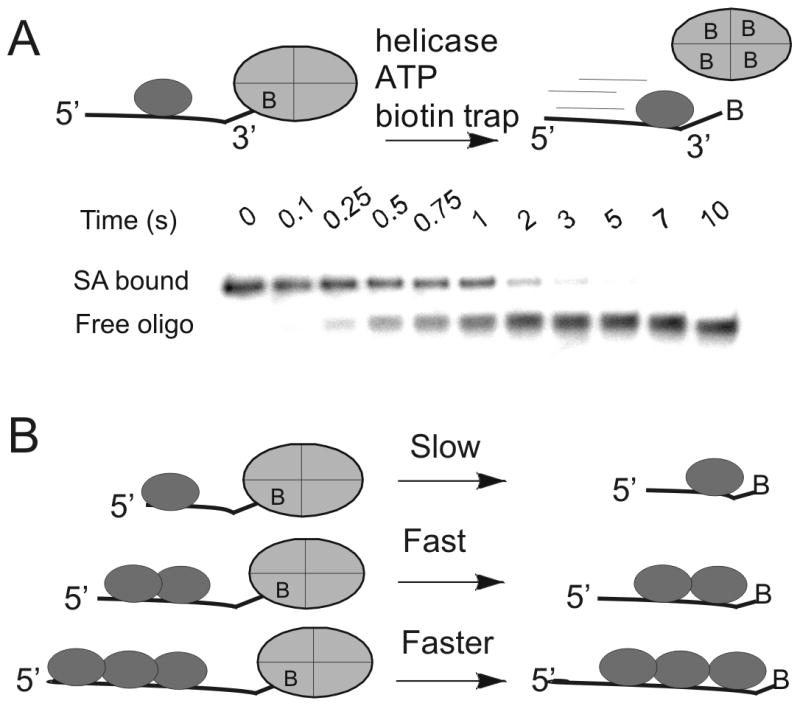
A. The action of a helicase on an oligonucleotide substrate can lead to rapid dissociation of streptavidin from biotin-labeled DNA. A native polyacrylamide gel can separate the streptavidin-bound DNA from free DNA. Helicases have been found capable of displacing streptavidin from either the 3′-end or the 5′-end, depending on the directional bias of the particular helicase. B) The rate of displacement of streptavidin from biotin-labeled DNA increases with increasing length of the ssDNA. In the case of Dda helicase, multiple molecules of the enzyme appear to function together by pushing in the same direction due to directionally biased translocation on ssDNA.
