Abstract
Polycythemia vera (PV) in children and adolescents is very rare. Data on clinical and laboratory evaluations as well as on treatment modalities are sparse. Here, we report the long-term clinical course of a PV patient first diagnosed more than 40 years ago at age 12. In addition, after a systematic review of the scientific medical literature, clinical and hematological data of 35 patients (19 female and 17 male) from 25 previous reports are summarized. Three patients developed PV following antecedent hematological malignancies. Budd–Chiari syndrome was diagnosed in seven patients indicating a particular risk of young patients of developing this disorder. One patient presented with ischemic stroke, one patient with gangrene, and three patients with severe hemorrhage. Three patients died from disease-related complications. Hematocrit levels and platelet counts were not correlated with disease severity. Leukocytosis >15×109/L was present in 9/35 patients and associated with a thromboembolic or hemorrhagic complication in seven patients. The few available data on molecular genetics and endogenous erythroid colony growth indicate changes comparable to those detectable in adult patients. Treatment varied enormously. It included aspirin, phlebotomy, hydroxycarbamide, busulfan, melphalan, pyrimethamine, and interferon-alpha. Two patients successfully underwent stem cell transplantation. Currently, it is impossible to treat an individual pediatric PV patient with an evidence-based regimen.
Keywords: Polycythemia vera, Childhood, Adolescence, Erythrocytosis, Budd–Chiari syndrome
Introduction
Erythrocytoses (synonymous to polycythemia or polyglobulia) constitute an extremely rare group of diseases in pediatric and juvenile patients. Primary erythropoietin (EPO)-independent erythrocytoses comprise congenital forms including primary familial and congenital polycythemia caused by EPO-receptor gene mutations and the acquired myeloproliferative disorder polycythemia vera (PV).
The incidence of PV is about 10–20/1,000,000, with a median age at presentation of 60 years. Only 1% of patients present before the age of 25, and only 0.1% are younger than 20 [1]. Therefore, very few pediatric PV patients have been reported to date. Systematic data on the clinical course, hematological characteristics, and on treatment modalities are sparse. Long-term follow-up observations from patients with manifestations of PV in childhood have not been reported to date.
Here, we present the results of an extensive review of the scientific medical literature to identify possible common clinical and hematological characteristics among pediatric patients with PV. In addition, we report the long-term clinical course of a female patient diagnosed more than 40 years ago at age 12 and first reported from by Dr. Wick in 1969 [2].
The patient
A female patient with PV diagnosed in childhood was followed for more than 40 years. This patient, who is now 54 years old, was first reported in 1969 [2]. The original report included the medical history from the first elevated erythrocyte count at age 2 to the presentation with stroke and right hemiparesis at the age of 12 and provided very detailed clinical and laboratory data. It was reported that after initial phlebotomies and short-term anticoagulation, two subsequent relapses led to the initiation of treatment with pyrimethamine (Daraprim), a folate antagonist, and of continuous anticoagulation with phenprocoumon. This treatment led to a stable hematological and clinical situation.
The clinical course following the initial report detailed below was gained from medical records kindly provided by the patient herself. Due to treatment failure manifested by the occurrence of a single focal seizure, pyrimethamine was withdrawn at age 17. For 2 years, phenylhydrazine treatment was attempted, but this was not well tolerated. Phlebotomy was re-initiated. At the age of 20, a single dose of radiophosphorus was administered. The patient developed a microembolism of the right eye at age 26 despite an apparently stable hematological situation and continuous treatment with phenprocoumon. Beginning 3 years later, the patient suffered from recurrent leg ulcers necessitating repeated surgical treatment. Antithrombotic prophylaxis was changed to heparin and later to low-molecular-weight heparin. The patient developed severe arterial hypertension. At age 46, the patient suffered a transient ischemic attack aggravating the pre-existing hemiparesis and causing an additional speech disorder. Shortly thereafter, splenectomy was performed since the massive splenomegaly apparently contributed to arterial hypertension by compressing the renal vessels and the left kidney. Because of subsequent thrombocytosis (2,000×109/L) treatment with hydroxycarbamide (HC) was started. A few months later, treatment was changed to busulfan, which was well tolerated, and led to a sufficient control of the hematological parameters even at low doses. Busulfan was withdrawn at age 52. At present, the patient is without specific treatment in a clinically and hematologically stable condition with hematocrit values around 0.45 and platelet counts below 400× 109/L. The patient is still suffering from residual neurological symptoms, particularly from motor deficits of the right hand. Treatment with low-molecular-weight heparin continues although the patient developed osteoporosis requiring medical treatment. The arterial hypertension is adequately treated.
Recent molecular analyses revealed typical findings including the presence of the JAK2V617F mutation, an increased CD177 messenger RNA (mRNA) expression, and the growth of EPO-independent endogenous erythroid colonies.
Review of published cases
A PubMed search (http://www.ncbi.nlm.nih.gov/entrez) was performed using the following terms:
(Children or pediatric or paediatric or childhood or child or familial) and (erythrocytosis or polycythemia or polycythaemia)
(Polycythemia or polycythaemia) and vera and (infancy or adolescence).
In addition, summarizing articles on patient groups defined either by age (“young patients”) or by a particular complication (Budd–Chiari syndrome) were evaluated for possible detailed data of individual patients.
The articles were regarded suitable for further evaluation if the reported patients met the Polycythemia Vera Study Group and/or World Health Organization (WHO) criteria. The following types of reports were considered:
All articles in English, German, or French language (if the journal was accessible)
Articles in another language but with a concise and detailed English abstract, including sufficient details on the patient. In some cases, it was possible to extract additional information from the original article
Articles not accessible and without detailed abstract but cited in other summaries with a sufficient amount of detailed data reported there.
Results and discussion
Thirty-six PV patients (19 female and 17 male) from 25 reports were evaluated for clinical and laboratory data [2–26]. Two recently published reports on markers of myeloproliferative diseases in a cohort of children and adolescents with PV comprising eight sporadic and five familial cases are discussed separately, since clinical data were limited and individual patient data were not presented [27, 28].
Age distribution
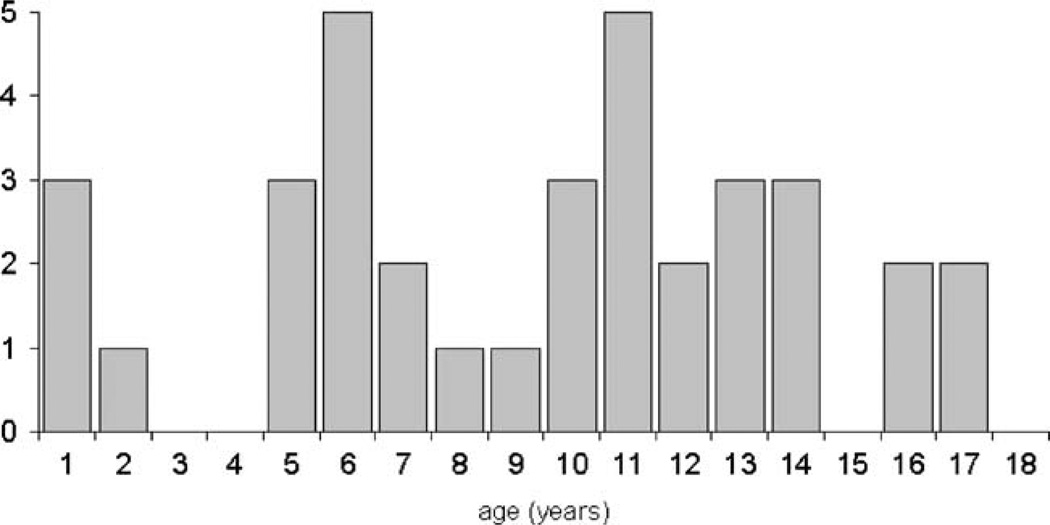
At onset of PV, the youngest patient was 7 months, the oldest was 17.5 years old (median age 11 years). The age distribution shows a first peak at the age of 5 to 6 years and a second at the prepubertal stage (10–14 years; Fig. 1). It is very difficult to find a reasonable explanation for the observed age distribution. In very young patients, diagnostic problems (e.g., misinterpretation of blood counts) might result in a late diagnosis in some cases thus leading to an accumulation of diagnosed cases at the preschool age. It is likewise conceivable that the onset of puberty precipitates the occurrence of clinical symptoms leading to the second peak.
Fig. 1.
Age distribution of pediatric patients with polycythemia vera
Clinical presentation and complications
PV in childhood and adolescence is not a mild disorder. Nine out of 36 patients (25%) developed severe thrombotic complications; three patients (8.3%) experienced severe bleeding events (hemorrhagic stroke, gastrointestinal hemorrhage, and post-dental extraction bleeding, Table 1). Three patients (8.3%) died from disease-related complications. About half of the patients were suffering from other symptoms probably related to PV.
Table 1.
Clinical complications and PV-related symptoms in pediatric patients
| Thrombotic and hemorrhagic complications/symptoms |
Before/at diagnosis of PV |
During follow-up |
|---|---|---|
| Budd–Chiari syndrome | 5 | 2 |
| Gangrene | 1 | |
| Stroke (thrombotic) | 1 | |
| Stroke (hemorrhagic) | 1 | |
| Pulmonary embolism (suspected) | 1 | |
| Gastrointestinal hemorrhage | 1 | |
| Post-dental extraction bleeding | 1 | 1 |
| Epistaxis | 2 | |
| Symptoms | ||
| Headache | 11 | 1 |
| Hypertension | 1 | 2 |
| Nausea | 3 | 1 |
| Syncope | 3 | |
| Lassitude | 3 | 1 |
| Dizziness | 2 | 1 |
| Pruritus | 3 | |
| Impaired vision | 2 | |
| Arthralgia | 1 | |
| Tinnitus | 1 |
The prevalence is given for every complication, thus, patients with more than one complication/symptom are counted for each of them. Events occurring before/at diagnosis and during follow-up in a single patient are listed only in the first column
Budd–Chiari syndrome was diagnosed in seven patients (19.4%) [7, 14, 19, 21, 25]. One patient died of chronic rejection and portal vein thrombosis following liver transplantation. A second patient died of progressive liver failure despite splenorenal shunt placement [14, 21]. One patient underwent orthotopic liver transplantation and has experienced 7 years of complication-free survival to date [25, 29]. Other patients were successfully treated with transjugular intrahepatic portosystemic shunting [7, 25]. Since we explicitly evaluated studies on Budd–Chiari syndrome for the inclusion of pediatric patients, the high prevalence may be influenced by a selection bias. However, the data are in agreement with several studies in young adult patients reporting a prevalence of Budd–Chiari syndrome of up to 30% [30, 31]. Thus, both pediatric and young adult PV patients apparently display a particular, currently unexplained predisposition to develop Budd–Chiari syndrome. The data from pediatric patients also confirm the particular predisposition of female patients to develop Budd–Chiari syndrome, previously described in adult patients with myeloproliferative disorders [32].
Two patients were reported to have suffered a stroke [2, 9]. One of these patients died of pneumonia after hemorrhagic stroke [9]. The long-term clinical course of the second patient is reported above and illustrates that the risk of thrombotic episodes as well as treatment complications accompany the patients for life.
Hematological presentation
The hematological presentation in children and adolescents with PV is heterogeneous (Table 2). Hematocrit values up to 0.80 have been reported [12].
Table 2.
Hematological data of pediatric patients at diagnosis of PV (n=number of informative patients)
| Median | Range | n | |
|---|---|---|---|
| Hemoglobin (g/dl) | 18.9 | 15.5–26.7 | 30 |
| Hematocrit (%) | 61 | 41–80 | 31 |
| Erythrocytes (×1012/L) | 7.6 | 5.2–11.2 | 27 |
| MCV (fl) | 76 | 62–95 | 14 |
| MCH (pg) | 24 | 18–35 | 9 |
| Reticulocytes (%) | 12 | 5–24 | 13 |
| Leucocytes (×109/L) | 13.2 | 3.3–22.2 | 32 |
| Platelets (×109/L) | 600 | 83–2,020 | 32 |
Leukocytosis emerges as an important predictive factor of thrombosis in untreated patients with myeloproliferative disorders [33]. For adult PV patients included in the European collaboration on low-dose aspirin in polycythemia vera trial, an increased risk of thrombosis, mainly of myocardial infarction, with leukocytes >15×109/L as compared to patients with leukocytes <10×109/L was reported [34]. A predictive leukocyte threshold of <9.5× 109/L in patients with essential thrombocythemia and PV was found in another recent study [35]. Previously reported pediatric PV patients generally presented with no or mild leukocytosis. At the time of diagnosis of PV, leukocytosis >15×109/L was present in eight patients (22%). Two of these patients presented with and one patient later developed Budd–Chiari syndrome [7, 21, 29]. Among the remaining 28 patients with leukocytes <15× 109/L, fourteen had initial leukocyte counts <10×109/L. Four of these patients later suffered from a serious thrombotic complication [2, 24, 25]. One of them had leukocytes >15×109/L at the subsequent presentation with Budd–Chiari syndrome [19]. Two patients with initial leukocytosis >15×109/L presented with severe post-dental extraction bleeding [12, 16]. One patient with an initially lower leukocyte count displayed leukocytosis >15×109/L at the time of hemorrhagic stroke [9]. Thus, in pediatric PV patients, the presence of marked to severe leukocytosis >15×109/L appears to be associated with both thrombotic and hemorrhagic complications. However, leukocytosis actually preceding the occurrence of a complication was documented in only one of the patients; whereas, it was detected at the time of the event in the remaining patients. Thus, it cannot be excluded that leukocytosis occurred secondary to either a thrombotic or a hemorrhagic event in at least some individuals. In contrast to a possible predictive value of the upper threshold of >15×109/L, the lower threshold of <9.5–10×109/L reported in adult PV patients does not seem to be predictive for a good clinical outcome in pediatric patients.
Thrombocytosis with platelet counts <400×109/L was found in 24 patients (66%). Thrombocytopenia (<150× 109/L) was present in four patients. Six patients had platelet counts >1,000×109/L, two of them at the time of presentation with Budd–Chiari syndrome, the remaining three, without severe symptoms. Three of the patients with hemorrhagic events presented with thrombocytosis, one patient with mild thrombocytopenia [4, 9, 12, 16]. Thus, thrombocytosis per se does not seem to be a major determinator for the clinical course in children and adolescents with PV.
Bone marrow trephine biopsies were performed in 31 patients (Table 3). Twenty-seven were reported with increased and three patients with normal cellularity. Erythropoiesis was increased in all patients. Myelopoiesis was increased in 13/19 patients. Seventeen of 23 informative patients also displayed an increased megakaryopoiesis with dysmorphic changes and clustering in some cases. Reduced bone marrow iron content was reported in 11/13 patients.
Table 3.
Bone marrow histology data of 31 informative pediatric PV patients (number of patients with reported hanges)
| Quantitative changes | Cellularity | Erythropoiesis | Myelopoiesis | Relation of Erythro- to Myelopoiesis | Megakaryopoiesis |
|---|---|---|---|---|---|
| ++ | 10 | 7 | 2 | 6 | |
| + | 17 | 13 | 11 | 10 | 11 |
| (+) | 3 | 1 | 1 | ||
| nl. | 3 | 5 | 5 | 6 |
++ severely,+ moderately, (+) mildly increased, nl. normal
Serum erythropoietin, endogenous erythroid colony growth, and molecular data
Serum EPO values were reported or commented in only 19 of 36 previously published patients. Two of them presented with normal serum EPO underlining that normal values do not exclude a diagnosis of PV [9, 29].
Apart from two recent studies, very few data on the examination of EPO-independent erythroid colony (EEC) growth and molecular genetics in pediatric PV patients are available. One of the recently published cohorts included eight sporadic and five familial pediatric PV cases [27, 28]. In this series, only four patients displayed EEC growth, three of eight patients an increased granulocyte CD177 (PRV-1) mRNA expression, only three patients had a JAK2V617F mutation, and none had a JAK2 exon 12 mutation.
In contrast, in a second study of eight patients, all examined for EECs were positive [25]. CD177 mRNA expression was elevated in three patients, normal in one, and within the borderline range in another patient. However, these two patients had a JAK2V617F mutation confirming the presence of PV at the molecular level. Similar cases have been described [36]. Overall, the JAKV617F mutation was found in six and a JAK2 Exon 12 mutation in two patients.
EPO-independent EEC growth was examined in eight other previously reported patients included in this review; six cultures were grown from peripheral blood and two from bone marrow [3, 12, 15, 18, 20, 26]. Only two additional patients were examined for molecular changes and presented with a JAK2V617F mutation [3, 26]. Interestingly, in one patient, the mutation was detected retrospectively in dried blood spots taken for postnatal metabolic screening at 2 days of age [26].
In conclusion, molecular changes in children and adolescents with PV are comparable to those detectable in adult patients. Thus, the recently proposed revised WHO diagnostic criteria [37] seem to be applicable also to children and adolescents with PV.
Treatment
Because of the long period during which the evaluated articles were published, treatment approaches varied enormously. They included phlebotomy as monotherapy (12 pts.) or in combination with other medical treatment (15 pts.). Other patients were treated with the alkylating agents melphalan and busulfan (2 pts.), radiophosphorus (1 pt.), or folate antagonists (2 pts.). HC was effective in four of seven patients [5, 22, 23, 29]. One patient treated with HC developed Budd–Chiari syndrome, in another patient, Budd–Chiari syndrome progressed despite HC therapy, and both patients died [14, 21]. Four patients have been treated with interferon-alpha [15, 17, 25]. Ten patients received low-dose acetylsalicylic acid usually in addition to other therapeutic interventions [14, 19, 21, 22, 25]. Stem cell transplantation was performed in three patients. Two patients were successfully treated with bone marrow transplantation from a matched sibling donor [20, 26]. One of them, the youngest patient so far reported, had progressive thrombocytosis during phlebotomy treatment; the other patient failed both phlebotomy and later HC therapy. Another patient had a successful stem cell transplantation from a matched unrelated donor after previous treatment with phlebotomy and interferon-alpha [38].
Due to the variety of treatment strategies in this small group of pediatric patients with PV, it is currently not possible to treat an individual patient with an evidence-based regimen. It thus seems reasonable to follow recommendations for young adult patients. In these patients, initial treatment in juvenile patients should comprise phlebotomies and low-dose aspirin. In young children, the potential risk of Reye’s syndrome should be considered although previous studies reported the predominant occurrence of this complication in children treated with high doses of acetylsalicylic acid. If any treatment apart from phlebotomy is required, interferon-alpha would certainly represent the preferable therapeutic agent. In the case of inadequacy of interferon treatment due to complications or treatment failure, stem cell transplantation even from an unrelated donor may be considered.
Secondary PV following antecedent hematological malignancy
Three of the 36 children and adolescents with PV had a history of malignancies prior to the manifestation of PV. The first patient was treated for acute lymphoblastic leukemia (ALL) at the age of 4. PV was diagnosed at age 10 [11]. A few months later, ALL relapsed. Interestingly, treatment for ALL relapse also led to control of the PV. The second patient presented with ALL at the age of 2 [21]. During ALL maintenance therapy, this patient developed transient isolated erythrocytosis. Three years after the end of chemotherapy (at the age of 7.5), the patient again presented with erythrocytosis and thrombocytosis indicating PV. Despite phlebotomy and HC treatment, this patient developed Budd–Chiari syndrome and died from progressive liver failure. The third patient is a girl with large-cell anaplastic lymphoma diagnosed at age 13 [25]. During routine follow-up after the end of lymphoma treatment, 2 years later, she presented with mild symptoms of dizziness, reported an episode of tinnitus, and occasional aquagenic pruritus as an initial manifestation of PV.
Although five reports on the concomitant presence of lymphoma and PV can be found [39–43], there are only two reports on patients with PV after lymphoma treatment [44, 45]. In addition, a young male patient (30 years) was reported who developed PV about 6 years after the successful treatment of acute myeloid leukemia [46].
The literature cites only very few published cases of “secondary” PV after treatment of hematological malignancies. It is thus remarkable that three of them are pediatric patients. Giving the large number of children and adults treated for either lymphoma or leukemia and the very low number of patients later affected by PV, the most reasonable explanation is coincidence. Nevertheless, the fact that this coincidence is found “predominantly” in children in whom PV per se is very rare makes it an interesting observation. Elucidation of a common predisposing alteration as well as characterization of the specific change initiating myeloproliferation in these patients might also contribute to a better understanding of the pathogenesis of “sporadic PV”.
Conclusion
PV in children and adolescents is a very rare. The single long-term follow-up, as well as the previous cases reported and summarized here, illustrates that PV in childhood and adolescence is a serious disorder. As demonstrated in this review, it is currently not possible to treat an individual patient with an evidence-based regimen. Thus, a close international co-operation of physicians and institutions is necessary to improve medical care for these patients and to elucidate the molecular etiology of pediatric PV. Open questions include the molecular event triggering early disease manifestation, the presumed particular predisposition of young patients to Budd–Chiari syndrome, as well as the apparent predominance of children among patients with the very rare event of secondary PV following antecedent hematological malignancy.
Contributor Information
Holger Cario, Email: holger.cario@uniklinik-ulm.de, Department of Pediatrics and Adolescent Medicine, University Hospital of Ulm, Eythstrasse 24, 89075 Ulm, Germany.
Mary Frances McMullin, Department of Haematology, Queen’s University Belfast, Belfast City Hospital, Belfast, UK.
Heike L. Pahl, Department of Anaesthesiology, University Hospital of Freiburg, Freiburg, Germany
References
- 1.Osgood EE. Polycythemia vera: age relationship and survival. Blood. 1965;26:243–256. [PubMed] [Google Scholar]
- 2.Wick H. Polycythaemia vera mit neurologischen Komplikationen bei einem 12jährigen Kind. Schweiz Med Wochenschr. 1969;99:186–189. [PubMed] [Google Scholar]
- 3.Park MJ, Shimada A, Asada H, Koike K, Tsuchida M, Hayashi Y. JAK2 mutation in a boy with polycythemia vera, but not in other pediatric hematologic disorders. Leukemia. 2006;20:1453–1454. doi: 10.1038/sj.leu.2404259. [DOI] [PubMed] [Google Scholar]
- 4.Aggeler PM, Pollycove M, Hoag S, Donald WG, Lawrence JH. Polycythemia vera in childhood. Studies of iron kinetics with Fe59 and blood clotting factors. Blood. 1961;17:345–350. [PubMed] [Google Scholar]
- 5.Berbis P, Devaux J, Benveniste MJ, Perrimond H, Privat Y. Severe erosive lichen planus and polycythemia vera in an adolescent. Dermatologica. 1987;174:244–248. doi: 10.1159/000249189. [DOI] [PubMed] [Google Scholar]
- 6.Cap J. Polycythemia vera in an 11-year-old child. Bone marrow depression after daraprim treatment. Cesk Pediatr. 1961;16:49–53. [PubMed] [Google Scholar]
- 7.Cobo F, Cervantes F, Garcia-Pagan JC, Bosch J, Rozman C, Montserrat E. Budd-Chiari syndrome associated with chronic myeloproliferative syndromes: analysis of 6 cases. Med Clin (Barc) 1996;107:660–663. [PubMed] [Google Scholar]
- 8.Crosato M, Castello D. Vaquez’s disease in childhood. Case report. Panminerva Med. 1964;105:62–68. [PubMed] [Google Scholar]
- 9.Danish EH, Rasch CA, Harris JW. Polycythemia vera in childhood: case report and review of the literature. Am J Hematol. 1980;9:421–428. doi: 10.1002/ajh.2830090409. [DOI] [PubMed] [Google Scholar]
- 10.Esposito L, Ferrara M. Polycythemia vera in childhood. Data on platelet function and blood coagulation factors. Pediatria (Napoli) 1976;84:208–223. [PubMed] [Google Scholar]
- 11.Hann HW, Festa RS, Rosenstock JG, Cifuentes E. Polycythemia vera in a child with acute lymphocytic leukemia. Cancer. 1979;43:1862–1865. doi: 10.1002/1097-0142(197905)43:5<1862::aid-cncr2820430540>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 12.Heilmann E, Klein CE, Beck JD. Primary polycythaemia in childhood and adolescence. Folia Haematol Int Mag Klin Morphol Blutforsch. 1983;110:935–941. [PubMed] [Google Scholar]
- 13.Marlow AA, Fairbanks VF. Polycythemia vera in an eleven-yr-old girl. N Engl J Med. 1960;263:950–952. doi: 10.1056/NEJM196011102631906. [DOI] [PubMed] [Google Scholar]
- 14.Melear JM, Goldstein RM, Levy MF, et al. Hematologic aspects of liver transplantation for Budd-Chiari syndrome with special reference to myeloproliferative disorders. Transplantation. 2002;74:1090–1095. doi: 10.1097/00007890-200210270-00006. [DOI] [PubMed] [Google Scholar]
- 15.Nagy K, Hunyadi K, Feher I, et al. Polycythemia vera in an 11-year old girl. Orv Hetil. 1996;137:27–30. [PubMed] [Google Scholar]
- 16.Natelson EA, Lynch EC, Britton HA, Alfrey CP. Polycythemia vera in childhood. A case with chromosomal abnormality, immunoglobulin deficiency, and chronic consumption coagulopathy. Am J Dis Child. 1971;122:241–244. [PubMed] [Google Scholar]
- 17.Olpinski M, Jakiela T, Korczowski R. Interferon alpha in the treatment of polycythemia vera in a 13-year old girl. Pediatr Pol. 1996;71:705–707. [PubMed] [Google Scholar]
- 18.Poggi V, Migliorati R, Fiore M, Amoroso R, Ghio R, Fiorillo A. Polycythemia vera: a new case report with onset in infancy. Haematologica. 1984;69:458–463. [PubMed] [Google Scholar]
- 19.Roth M, Haag K, Krause T, Blum U, Hellerich U. Aszites und Splenomegalie im Kindesalter. Med Klin. 1990;85:529–532. [PubMed] [Google Scholar]
- 20.Stobart K, Rogers PC. Allogeneic bone marrow transplantation for an adolescent with polycythemia vera. Bone Marrow Transplant. 1994;13:337–339. [PubMed] [Google Scholar]
- 21.Sutherland ND, Gonzalez-Peralta R, Douglas-Nikitin V, Hunger SP. Polycythemia vera in a child following treatment for acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2004;26:315–319. doi: 10.1097/00043426-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Turker M, Ozer EA, Oniz H, Atabay B, Yaprak I. Polycythemia vera in a 12-year-old girl: a case report. Pediatr Hematol Oncol. 2002;19:263–266. doi: 10.1080/08880010252899433. [DOI] [PubMed] [Google Scholar]
- 23.Vodoff MV, Nelken B, Vic P, Farriaux JP. Treatment with hydroxyurea of polycythemia vera in a 11 year old girl. Arch Pediatr. 1996;3:870–873. doi: 10.1016/0929-693x(96)87575-0. [DOI] [PubMed] [Google Scholar]
- 24.Kovalev Iu R, Erman LV. Polycythemia vera in children. Probl Gematol Pereliv Krovi. 1977;22:52–55. [PubMed] [Google Scholar]
- 25.Cario H, Schwarz K, Herter JM, et al. Clinical and molecular characterisation of a prospectively collected cohort of children and adolescents with polycythemia vera. Br J Haematol. 2008;142:622–626. doi: 10.1111/j.1365-2141.2008.07220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly K, McMahon C, Langabeer S, Eliwan H, O’Marcaigh A, Smith OP. Congenital JAK2V617F polycythemia vera: where does the genotype-phenotype diversity end? . Blood. 2008;112:4356–4357. doi: 10.1182/blood-2008-08-175620. [DOI] [PubMed] [Google Scholar]
- 27.Teofili L, Giona F, Martini M, et al. Markers of myeloproliferative diseases in childhood polycythemia vera and essential thrombocythemia. J Clin Oncol. 2007;25:1048–1053. doi: 10.1200/JCO.2006.08.6884. [DOI] [PubMed] [Google Scholar]
- 28.Teofili L, Giona F, Martini M, et al. The revised WHO diagnostic criteria for Ph-negative myeloproliferative diseases are not appropriate for the diagnostic screening of childhood polycythemia vera and essential thrombocythemia. Blood. 2007;110:3384–3386. doi: 10.1182/blood-2007-06-094276. [DOI] [PubMed] [Google Scholar]
- 29.Cario H, Pahl HL, Schwarz K, et al. Familial polycythemia vera with Budd-Chiari syndrome in childhood. Br J Haematol. 2003;123:346–352. doi: 10.1046/j.1365-2141.2003.04591.x. [DOI] [PubMed] [Google Scholar]
- 30.Najean Y, Mugnier P, Dresch C, Rain JD. Polycythaemia vera in young people: an analysis of 58 cases diagnosed before 40 years. Br J Haematol. 1987;67:285–291. doi: 10.1111/j.1365-2141.1987.tb02349.x. [DOI] [PubMed] [Google Scholar]
- 31.Perea G, Remacha A, Besses C, Jimenez M, Florensa L, Cervantes F. Is polycythemia vera a serious disease in young adults? Haematologica. 2001;86:543–544. [PubMed] [Google Scholar]
- 32.De Stefano V, Teofili L, Leone G, Michiels JJ. Spontaneous erythroid colony formation as the clue to an underlying myeloproliferative disorder in patients with Budd-Chiari syndrome or portal vein thrombosis. Semin Thromb Hemost. 1997;13:411–418. doi: 10.1055/s-2007-996117. [DOI] [PubMed] [Google Scholar]
- 33.Barbui T, Carobbio A, Rambaldi A, Finazzi G. Perspectives on thrombosis in essential thrombocythemia and polycythemia vera: is leukocytosis a causative factor? Blood. 2009 doi: 10.1182/blood-2009-02-206797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109:2446–2452. doi: 10.1182/blood-2006-08-042515. [DOI] [PubMed] [Google Scholar]
- 35.Caramazza D, Caracciolo C, Barone R, et al. Correlation between leukocytosis and thrombosis in Philadelphia-negative chronic myeloproliferative neoplasms. Ann Hematol . 2009 doi: 10.1007/s00277-009-0706-x. [DOI] [PubMed] [Google Scholar]
- 36.Lippert E, Boissinot M, Kralovics R, et al. The JAK2–V617F mutation is frequently present at diagnosis in patients with essential thrombocythemia and polycythemia vera. Blood. 2006;108:1865–1867. doi: 10.1182/blood-2006-01-013540. [DOI] [PubMed] [Google Scholar]
- 37.Tefferi A, Thiele J, Orazi A, et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert panel. Blood. 2007;110:1092–1097. doi: 10.1182/blood-2007-04-083501. [DOI] [PubMed] [Google Scholar]
- 38.Reinhard H, Klingebiel T, Lang P, Bader P, Niethammer D, Graf N. Stem cell transplantation for polycythemia vera. Pediatr Blood Cancer. 2008;50:124–126. doi: 10.1002/pbc.20906. [DOI] [PubMed] [Google Scholar]
- 39.Cottrill C, Geller A, diSpaltro FX, Weissglass B, Klainer AS, Bisaccia E. Control of polycythaemia vera with photo-hemotherapy in a patient with cutaneous T-cell lymphoma. Br J Haematol. 1994;86:225–226. doi: 10.1111/j.1365-2141.1994.tb03286.x. [DOI] [PubMed] [Google Scholar]
- 40.Heinle EW, Jr, Sarasti HO, Garcia D, Kenny JJ, Westerman MP. Polycythemia vera associated with lymphomatous diseases and myeloma. Arch Intern Med. 1966;118:351–355. [PubMed] [Google Scholar]
- 41.Khojasteh A, Perry MC. Coexistence of diffuse lymphocytic lymphoma and polycythemia vera. South Med J. 1981;74:771–772. doi: 10.1097/00007611-198106000-00038. [DOI] [PubMed] [Google Scholar]
- 42.Rizzi R, Liso A, Pannunzio A, Carluccio P, Specchia G, Liso V. Concomitant primary polycythemia vera and follicle center cell non-Hodgkin lymphoma: a case report and review of the literature. Leuk Lymphoma. 2002;43:2217–2220. doi: 10.1080/1042819021000016113. [DOI] [PubMed] [Google Scholar]
- 43.Stolinsky DC. Twelve-year remission of polycythemia vera following Hodgkin’s disease and chemotherapy. CA Cancer J Clin. 1981;31:57–60. doi: 10.3322/canjclin.31.1.57. [DOI] [PubMed] [Google Scholar]
- 44.Bosch F, Cervantes F, Lopez-Guillermo A, Carreras E, Montserrat E, Rozman C. Polycythaemia vera following non-Hodgkin’s lymphoma. Leuk Lymphoma. 1992;8:501–502. doi: 10.3109/10428199209051034. [DOI] [PubMed] [Google Scholar]
- 45.Harrison P, Neilson JR, Lumley MA, Milligan DW. Development of polycythaemia rubra vera following treatment for centroblastic lymphoma. Acta Haematol. 1996;96:113–114. doi: 10.1159/000203729. [DOI] [PubMed] [Google Scholar]
- 46.Chabannon C, Bost M, Hollard D. A case of polycythemia vera occurring in a patient with acute non-lymphoblastic leukemia (ANLL) in long-term first complete remission. Leukemia. 1994;8:1243–1244. [PubMed] [Google Scholar]