Abstract
Esophageal atresia with or without tracheoesophageal fistula (EA/TEF) is a relatively common birth defect often associated with additional congenital anomalies such as vertebral, anal, cardiovascular, renal and limb defects, the so-called VACTERL association. Yet, little is known about the causal genetic factors. Rare case reports of gastrointestinal anomalies in children with triple X syndrome prompted us to survey the incidence of structural and numerical changes of chromosome X in patients with EA/TEF. All available (n=269) karyotypes of our large (321) EA/TEF patient cohort were evaluated for X-chromosome anomalies. If sufficient DNA material was available, we determined genome-wide copy number profiles with SNP array and identified subtelomeric aberrations on the difficult to profile PAR1 region using telomere-multiplex ligation-dependent probe amplification. In addition, we investigated X-chromosome inactivation (XCI) patterns and mode of inheritance of detected aberrations in selected patients. Three EA/TEF patients had an additional maternally inherited X chromosome. These three female patients had normal random XCI patterns. Two male EA/TEF patients had small inherited duplications of the XY-linked SHOX (Short stature HOmeoboX-containing) locus. Patients were small for gestational age at birth (<P5) and had additional, mostly VACTERL associated, anomalies. Triple X syndrome is rarely described in patients with EA/TEF and no duplications of the SHOX gene were reported so far in these patients. As normal patterns of XCI were seen, overexpression of X-linked genes that escape XCI, such as the SHOX gene, could be pathogenic by disturbing developmental pathways.
Keywords: esophageal atresia, tracheoesophageal fistula, triple X syndrome, SHOX duplication, VACTERL association
INTRODUCTION
Esophageal atresia (EA) with or without tracheoesophageal fistula (TEF) is a relatively common birth defect affecting approximately 1:3500 newborns. These newborns can have a heterogeneous phenotype, some have EA and/or TEF as an isolated defect and others have more anomalies, predominantly VACTERL (vertebral, anal, cardiovascular, tracheoesophageal, renal and limb) associated.1
EA/TEF is a variable feature in several genetic syndromes, for example, Feingold (MYCN), CHARGE (CHD7), Anophthalmia-Esophageal-Genital (AEG) syndrome (SOX2) and Fanconi anemia.2 In addition, the genetic defects in these syndromes and other putative causal genetic aberrations are described in EA/TEF patients. Although there are chromosomal hotspots, these aberrations are mostly scattered across the genome.2
Structural and numerical chromosome abnormalities affecting sex chromosomes have been described in patients with congenital malformations.3, 4, 5 These defects are rare in patients with EA/TEF, although EA/TEF is a variable feature in patients with Opitz G syndrome (MID1) and VACTERL association with hydrocephalus (FANCB).1, 2
There are several reports describing X-chromosome duplication in association with gastrointestinal anomalies.6 This prompted us to evaluate retrospectively the cytogenetic results in our EA/TEF cohort.
We identified three patients with a triple X karyotype, strengthening the relationship of gastrointestinal anomalies and X-chromosome triplication. In addition to classical karyotyping, we examined the patient DNA with telomere-multiplex ligation-dependent probe amplification (MLPA) and microarrays for structural X-chromosome abnormalities. These molecular–genetic studies revealed Short stature HOmeoboX-containing gene (SHOX) duplications in two additional patients with EA, TEF and limb anomalies.
We hypothesize that genes on X and/or genes that escape X-chromosome inactivation (XCI) could influence essential developmental pathways in limb and foregut development.
PATIENTS AND METHODS
Patient population
This study was approved by the Medical Ethical Review Board of Erasmus MC – Sophia Children's Hospital. After retrieving (parental) informed consent, 321 patients with EA/TEF, admitted to the Department of Paediatric Surgery, were included. Pregnancy, clinical and follow-up data were extracted from medical charts. Available DNA and cell lines of patients (n=180) and parents were collected and used for genetic analysis. Patients with a previous confirmed genetic syndrome, known chromosomal anomaly and/or pathogenic point mutation were excluded from further molecular–genetic evaluation. There is a weak evidence for an association of EA/TEF with certain environmental components;7 however, we did not exclude any of the patients in our cohort based on these risk factors.
The database (>100 000 patients) of prenatal and postnatal diagnostics of our Department of Clinical Genetics was searched for triple X karyotypes and confirmed SHOX duplications.
Cytogenetic evaluation
Karyotyping was performed according to standard protocols on either lymphocytes from peripheral blood cultures or after amniocentesis. Karyotyping had been performed for 269 of the 321 patients of our cohort, as systematic cytogenetic follow-up of patients with congenital anomalies was not carried out before 1998.
MLPA and quantitative PCR
Not all array chips used had sufficient marker density in the PAR1 region. Therefore, we additionally screened for copy number variations in this region with MLPA, using the P036E1 and P070A2 Salsa Telomere Kit (MRC Holland, Amsterdam, The Netherlands) as described previously.8 Genemarker 1.6 (SoftGenetics; LLC, State College, PA, USA) was used for data analysis. If duplications of the PAR1 region were detected, copy number profiling of the SHOX region was confirmed with a newly developed qPCR assay, with 11 amplicons within SHOX and the PAR1 region.9
Fluorescent in situ hybridization
The target BAC clone for Xp22 (RP11-800K15) and control probes on Xq25 (RP11-49N19) and 8p12 (RP11-489E7) were selected from the University of Santa Cruz (UCSC) genome browser (UC Santa Cruz, Santa Cruz, CA, USA; assembly March 2006) and ordered from BACPAC Resources (Children's Hospital of Oakland Research Institute, Oakland, CA, USA). After isolation of the BAC DNA, the probes were labeled and used for fluorescent in situ hybridization (FISH) on chromosome preparations from patients and parents, according to standard protocols.10
RNA-FISH analysis and immunocytochemistry of human cell lines
RNA-FISH and immunocytochemistry were performed on fibroblast cell lines (>90% confluence) and EBV-transformed lymphocytes, as previously described by Jonkers et al11, 12 using a 16.4 kb plasmid covering the complete XIST RNA sequence as described previously.13, 14
HUMARA analysis
To determine the parental origin and the methylation status of the additional X chromosome, we used the HUMARA assay (human methylation of the androgen receptor assay) using 40 ng of genomic DNA input for the digestion reaction and gel electrophoresis to separate PCR products.15
Microarrays
The genome-wide copy number profile of all patients (n=180) was determined using either Affymetrix GeneChip Human Mapping 250K NSP1 (Affymetrix, Santa Clara, CA, USA), HumanQ610, HumanCytoSNP-12v1 to 2.1 or HumanOmniExpress (Illumina, San Diego, CA, USA). We generated Affymetrix CEL files with the Affymetrix genotype command console v.3.2 software (Affymetrix). The HumanCytoSNP-12v2.1 chip (Illumina) was used in the cases with SHOX duplications and their parents, for better coverage of the PAR1 region. All procedures were carried out according to the manufacturer's protocol as described previously.16
SALL1 mutation analysis
Sequencing of the coding region of the SALL1 gene, including the splice sites, was carried out as described previously.17 Primer sequences are available on request. We did not have sufficient DNA of triple X patient 3 to perform additional Sanger sequencing.
Statistical analysis
Differences in two proportions were tested with the Pearson's χ2-test, reported with a 95% confidence interval (CI), performed in SPSS 15.0 (IBM, Armonk, NY, USA).
RESULTS
Patient characteristics
Patients included in the Erasmus MC – Sophia Children's Hospital EA/TEF cohort can be subdivided into four categories: isolated EA and/or TEF (45%), patients with one additional core VACTERL component (27%), VACTERL association, for example, three or more of the VACTERL core components (21%) and patients with a diagnosed genetic syndrome (7%).18, 19
Triple X syndrome
A triple X karyotype (see Supplementary Figure 1) was identified in three EA/TEF patients, resulting in an odds ratio for triple X syndrome of 11.3 (95% CI=3.6–35.2). All three were small for gestational age at birth (<5th percentile), with maternal ages ranging from almost 26 to 28 years. The first patient had all VACTERL features and additional genitourinary anomalies. The second patient had TEF and mild dysmorphic features and the third patient had EA/TEF, ventricular septal defect and thin fingers (patients 1–3; Table 1). SNP array analysis performed to exclude copy number variations elsewhere in the genome did not reveal additional possible pathogenic copy number variations in any of the three triple X patients.
Table 1. Congenital anomalies of EA/TEF patients with X-chromosome anomaly.
| Pt. no. | Karyotype | MLPA results | Maternal age | Clinical features |
|---|---|---|---|---|
| 1 | 47,XXX | X/Yp SHOX enh | 25.9 | Absent sacrum Anal atresia Pulmonary stenosis EA+TEF Vesicourethral reflux; urethral atresia Absent thumbs Cloacal malformation: abnormal labia, hydrometrocolpos |
| 2 | 47,XXX | X/Yp SHOX enh | 28.2 | TEF Dysmorphic features |
| 3 | 47,XXX | X/Yp SHOX enh | 26.1 | EA+TEF Ventricular septum defect Thin fingers |
| 4 | 46,XY | X/Yp SHOX enh paternal inheritance | 31.5 | Aberrant subclavian artery EA+TEF Horseshoe kidneys Adducted thumbs; left thumb smaller than right Hypospadias Frontal bossing Dysmorphic features |
| 5 | 46,XY | X/Yp SHOX enh maternal inheritance | 34.3 | Atrial septum defect (type II) EA+TEF Proximal placement of thumbs |
Abbreviations: EA, esophageal atresia; enh, enhanced signal with MLPA kits P036E1 and P070A2; TEF, tracheoesophageal fistula.
Searching the database of our Department of Clinical Genetics yielded 59 non-mosaic 47,XXX karyotypes since 1988: 29 had been detected prenatally and 30 postnatally (apart from the above three patients). Indications (not mutually exclusive) for prenatal karyotyping were: maternal age >35 years (n=25), congenital malformations on ultrasound (n=4), increased risk for Down syndrome on first trimester screening ultrasound (n=2), increased echogenicity of the fetal bowel on ultrasound (n=1) and congenital anomalies in an earlier pregnancy (n=3). One screening, with an increased maternal age indication, concerned a twin pregnancy, of which one sib had a 47,XXX karyotype. Postnatal patients were karyotyped based on the following indications: suspicion of Fragile X syndrome (n=5), mental retardation (n=5), multiple congenital anomalies (n=4), combined mental retardation and multiple congenital anomalies (n=3), repeating spontaneous abortions in the index (n=4), failure to thrive (n=4), a chromosomal abnormality in the family (n=3), suspicion of trisomy 21 (n=1) and a possible chromosomal aberration in juvenile systemic lupus erythematosus (n=1).
Postnatal follow-up of 13 pregnancies was not documented. Seven pregnancies were terminated in one case because the fetus showed anencephaly. Congenital malformations had been documented for three of the nine births: a neural tube defect, hygroma colli with generalized edema and osteogenesis imperfecta, respectively. A review of the medical charts of the postnatally diagnosed patients identified at least four patients with congenital heart defects.
X-inactivation studies
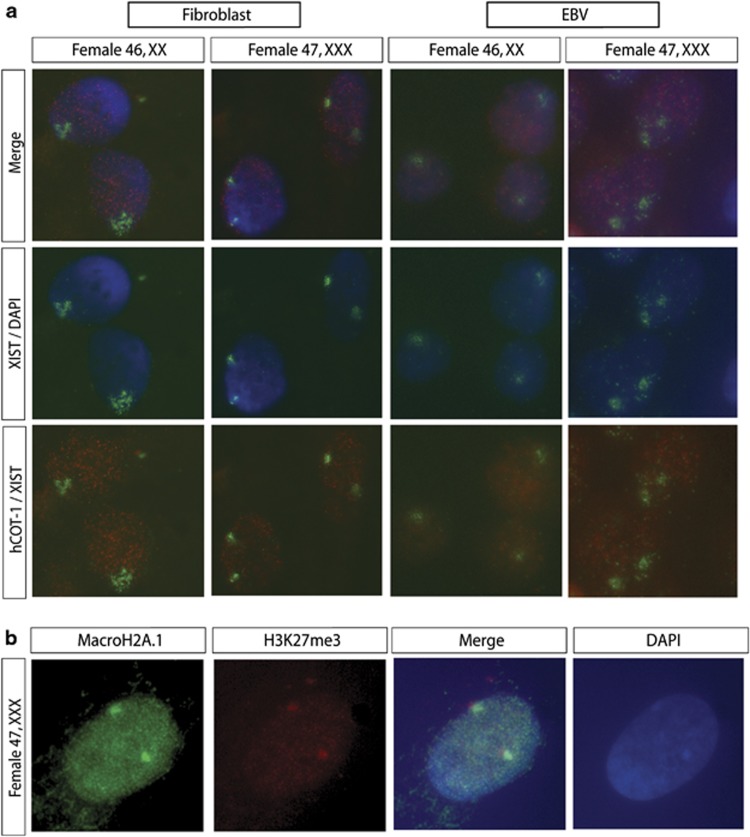
Previous studies have indicated that only one X-chromosome remains active in somatic cells of 47,XXX patients. RNA-FISH and immunocytochemistry were performed to assess the X-inactivation status in cell lines derived from the 47,XXX patients of our cohort. RNA-FISH analysis revealed two XIST clouds in almost every fibroblast and lymphocyte cell (>95% of the nuclei), colocalizing with an area of low-level COT-1 expression (Figure 1a). Immunocytochemistry of 47,XXX fibroblasts detected enrichment of the facultative heterochromatin markers H3K27me3 and MacroH2A1 on two X-chromosomes, colocalizing with the DAPI-dense Barr bodies (Figure 1b). HUMARA analysis confirmed a maternal origin of the additional X chromosome in all three patients. In all these patients and their mothers random XCI was observed, with no skewed preference of inactivation of a particular X-chromosome (Supplementary Figure 3).
Figure 1.
XCI studies in triple X patients. (a) RNA-FISH analysis detecting XIST and COT-1 RNA. XIST RNA was detected using a digeoxin-labeled cDNA probe12, 14 (fluorescein isothiocyanate (FITC), green), and a biotin-labeled COT-1 DNA probe detected expression of repetitive sequences (rhodamine, red). Characteristics for an inactive X-chromosome are the presence of XIST RNA accumulation and absence of COT-1 RNA. In 47,XXX patient cells, two XIST clouds and COT-1 holes were detected in the majority of the cells (>95% of the nuclei, n>100). Nuclei were counterstained with 4',6-diamidino-2-phenylindole (DAPI). (b) Immunocytochemistry detecting H3K27me3 (rhodamine, red) and MacroH2A1 (FITC, green). Enrichment of H3K27me3 and MacroH2A1 was found on two X chromosomes in 47,XXX fibroblast cell lines, colocalizing with DAPI-dense Barr bodies, indicative of two inactive X-chromosomes. Nuclei are counterstained with DAPI.
SHOX duplications
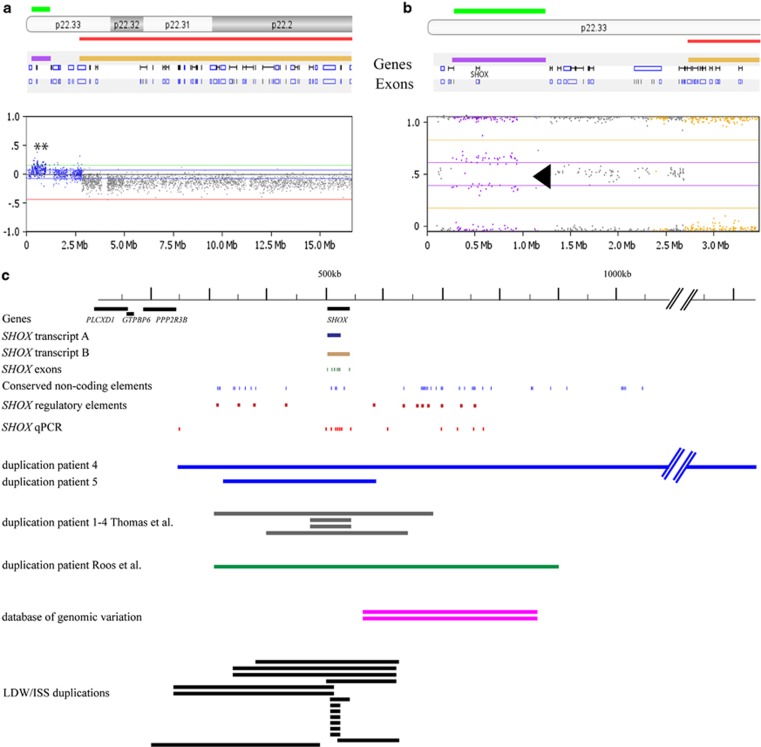
Enrichment of the triple X karyotype in our EA/TEF cohort prompted us to look further in our cohort for sex-chromosome aberrations with microarray and telomere-MLPA. These results indicated inherited SHOX duplications in two boys: patient 4 with a paternally inherited partial PAR1 duplication and patient 5 with a maternally inherited partial PAR1 duplication. Duplication of the SHOX gene is a rare event, it is only present twice in the database of genomic variation. Both SHOX duplications were confirmed with a SHOX-specific qPCR9 (Figure 2a and Supplementary Figure 2).
Figure 2.
Triple X syndrome and SHOX aberrations in patients with esophageal atresia/tracheoesophageal fistula (OA/TOF). (a) The HumanCytoSNP-12 chip (Illumina) showing an interstitial duplication of the PAR1 region (base pair position chrX: 248 968–1 229 976), including the SHOX gene in patient 4. The elevated log 2 ratio indicates the duplicated segment (green bar). (b) The HumanCytoSNP-12 chip result visualized in Biodiscovery Nexus CN6.1. The B-allele frequency of patient 4 is indicated in the enlarged right panel (purple bar, arrow). The shift from a heterozygous state (gray dots, 0.5) to a 0.33/0.66 frequency (purple dots) is indicative for a copy number change. Taken together with the raise in the log R, this state is indicative for a copy number gain. (c) Schematic presentation of the SHOX A and B transcripts (dark blue and brown), the conserved noncoding elements (blue),20 SHOX gene exons (green), SHOX qPCR (red)9 and SHOX regulatory elements described in literature (dark red), the SHOX duplications in patients 4 and 5 and their position compared the duplications observed in patients 1–4 in Thomas et al,49 Roos et al,48 the database of genomic variation and the duplication in LWD/ISS patients described by Benito-Sanz et al.43 The red lines indicate the length of the duplications, with a larger duplication in patient 4 (amplicons 1–13) and a SHOX duplication in patient 5 (amplicons 2–8) (Supplementary Figure 2). Both MLPA probes (orange) were duplicated in patients 4 and 5.
To exclude additional potential pathogenic copy number variations in other regions in the genome of both patients and their parents, we analyzed their genome-wide copy number profile. These results revealed multiple copy number variations in both the patients and their parents. However, upon closer examination of these regions in the database of genomic variation, all of them were common polymorphisms. Given their relative high population frequency, they were not considered as potential pathogenic copy number variations in a relatively rare condition as EA/TEF or VACTERL association.
Patient 4's twin sib was spontaneously aborted in the third month of gestation. His mother was diagnosed with Goldenhar syndrome. She had right-sided hemifacial microsomia, anotia, deafness, paresis of the pallatum molle and facial dysmorphisms. Two relatives on the mother's side had thumb anomalies. EA/TEF or other major anomalies were absent in the father. The boy was small for gestational age at birth (<2 SD) and had associated cardiovascular, renal, limb and genital anomalies (Table 1).
SHOX-specific qPCR confirmed the duplication of the SHOX gene (amplicons 1–13) in the PAR1 region in patient 4 and his father. Cultured lymphocytes of proband or parents were not available for FISH validation or to localize the duplication. The 981 kb duplicated segment corresponds to the PAR1 region on chromosome X, and both SHOX variants, SHOXA g1-292dup and SHOXB g1-225dup, were completely duplicated in patient 4, including all of its regulatory sequences20, 21, 22 (arr [hg18] Xp22 or Yp11(248 968–1 229 976) × 3) (Figures 2a and b and Supplementary Figure 4).
Patient 5, with apparently healthy parents, was also small for gestational age at birth and had cardiac and limb anomalies. He inherited a PAR1 duplication/suspected rearrangement from his mother overlapping the SHOX gene (arr [hg18] Xp22 (325 941–593 267) × 3) and separated by a region with normal copy numbers, a second Xp22 duplicated segment containing seven other genes (IL3RA, SLC25A6, ASMTL, PP1164, P2RY8, SFRS17A and ASMT) (arr [hg18] Xp22(1 428 051–1 891 174) × 3; Supplementary Figure 5). SHOX-specific qPCR (duplication of amplicons 2–8) confirmed the SHOX gene duplication in patient 5 and his mother. Moreover, FISH (BAC clone RP11-800K15) confirmed the location of the duplication at chromosome band Xp22 (see Supplementary Figure 6). The direction of the inserted duplicated segment was not determined.
SALL1 mutation analysis
Mutation analysis of the SALL1 coding region only identified common variants, for example, two missense variants, one in all patients and a control (rs4614723, minor allele frequency is 1.514%) and one in patient 4 (rs13336129, minor allele frequency is 6.651%). We also identified one intronic variant in patient 4 (rs13336129, minor allele frequency is 45.545%) and two synonymous variants in triple X patient 1 and a control (rs11645288, minor allele frequency is 17.816% and rs1965024, minor allele frequency is 49.216%).
DISCUSSION
Triple X syndrome and gastrointestinal anomalies
Congenital malformations and mental retardation syndromes have been linked to the X-chromosome. Our search for genes or loci involved in EA/TEF or other foregut-related anomalies identified five patients in our cohort with chromosome X/Yp aberrations: three with triple X syndrome and two with inherited PAR1 duplications.
A large proportion of triple X females have a subclinical phenotype; therefore, a triple X karyotype is usually a random finding in prenatal screening or cytogenetic follow-up of pregnancies. Most 47,XXX females remain undiagnosed; therefore, triple X syndrome has an estimated incidence rate of 0.10% with an average maternal age of 33.4 Affected girls have a lower birth weight, more accelerated growth until puberty, long legs and an increase in behavioral problems and psychiatric disorder prevalence.4 In 90% of patients, the additional X chromosome is the result of a maternal meiotic I error, and the incidence of non-disjunction errors increases with maternal age.23
Sex chromosome triploidies (47,XXX/47,XXY/47,XYY) are a rare (0.42%) finding in a large (n=4282) prenatal cohort analyzed with karyotyping and microarray.24 Haverty et al25 calculated the incidence rate in female subjects and found it to be 0.17%. In a recent European study of the EUROCAT working group, 0.054 triple X patients/1000 births were observed.26 In our EA/TEF cohort, the incidence rate of triple X syndrome is 1.12%, with an average maternal age of 30.8 years, which is 11 times higher than that in the estimated general population and 6.5 times higher than in the calculated incidence rate by Haverty et al.25 Guichet et al27 reviewed prenatally and postnatally diagnosed 47,XXX karyotypes from 18 laboratories. Mental retardation or congenital malformations were described in over one-third of the 190 patients reported. In all cases, weight-for-gestational-age at birth was under the 25th percentile. Congenital anomalies associated with the triple X syndrome described in case reports include anomalies of the urinary tract, genital anomalies and craniofacial anomalies, especially a reduced head circumference and/or decreased brain volume.4, 28, 29 Genitourinary malformations are well described in triple X patients. They often are associated with lower gastrointestinal tract anomalies; perhaps, a cloacal septation problem gives rise to the higher incidence of these types of malformations. Gastrointestinal anomalies, including atresia of the esophagus and duodenum and jejunum as well as omphalocele and anorectal malformations, have been reported sporadically.6, 25, 30, 31, 32, 33, 34, 35, 36 All 13 reported patients with gastrointestinal and/or foregut-related anomalies are reviewed in Table 2. The patient described by Hoang et al32 shows a similar phenotype compared to our patients: higher mesodermal defects (EA) and lower mesodermal defects (anal atresia, genitourinary defects).
Table 2. Congenital malformations of the gastrointestinal tract and/or foregut-related structures in triple X syndrome.
| N | Genital | Urinary | Gastrointestinal anomalies | V | A | C | TE | R | L | Other anomalies | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | + | + | Cloacal extrophy incl. imperforate anus, esophageal atresia+tef | + | + | + | + | + | Dysmorphic features | Present study | |
| 1 | Jejunal atresia | 34 | |||||||||
| 1 | Duodenal atresia | 35 | |||||||||
| 1 | Duodenal atresiaa | + | 6 | ||||||||
| 1 | Omphalocele | Beckwith–Wiedemann syndrome | 36 | ||||||||
| 1 | Omphalocele | + | 31 | ||||||||
| 1 | + | + | + | Pulmonary hypoplasia, laryngeal atresia, craniofacial anomalies | 56 | ||||||
| 1 | Ectopic anus | + | + | Clinodactyly, inferior coloboma, clinodactyly, dysmorphic features | 33 | ||||||
| 1 | + | + | Cloacal extrophy incl. imperforate anus | + | 25 | ||||||
| 1 | + | + | Cloacal extrophy incl. imperforate anus and rectoperineal fistula, colonic atresia, omphalocele | + | + | 30 | |||||
| 1 | + | + | Imperforate anus, esophageal atresia+TEF | + | + | + | + | Pulmonary hypoplasia, agenesis of gallbladder | 32 |
Abbreviations: A, anorectal malformations; C, cardiovascular anomalies; GI, gastrointestinal malformations; L, limb malformations; N, number of patients; R, renal anomalies; TE, esophageal atresia and/or tracheoesophageal fistula; TEF, tracheoesophageal fistula; V, vertebral defects.
Duodenal atresia due to annular pancreas.
X-inactivation patterns
The HUMARA assay demonstrated the maternal origin of the supernumerary X-chromosome and the absence of skewed preference for a particular X chromosome in our triple X patients. RNA-FISH analysis and immunocytochemistry demonstrated inactivation of two out of three X-chromosomes.
Overexpression of genes escaping X-inactivation could be responsible for the phenotypical abnormalities observed in our three EA/TEF patients and the gastrointestinal patients described in literature. Ten percent of genes have a variable pattern of gene X-inactivation and expression.37 The extent of this escape is tissue specific, and often results in variable or lower levels of expression from the inactive X-chromosome compared with the active X-chromosome.38, 39 Why and how certain genes escape XCI, especially in humans, is still unknown.40 Female ‘escapees' may have a dosage-sensitive function, which would explain the phenotype in patients with sex chromosome anomalies, such as Turner and Klinefelter syndromes. The observation that 47,XXX females also have decreased brain volume in the presence of normal pubertal maturation suggests a possible direct dosage effect of X-chromosomal genes.41 Two EA candidate genes MID1 and FANCB do not escape X-inactivation, although there is a X-inactivation preference for the X chromosome that contains the mutated allele in Fanconi anemia.24 Other genes that escape XCI could perhaps cause the gastrointestinal anomalies found in our cohort.
SHOX duplications
One of those escapees, located in the PAR1 region, is SHOX. This gene has two isoforms: SHOXA and SHOXB, which are surrounded by several conserved noncoding regulators. 20 SHOX encodes a cell-specific homeodomain protein, and isoform A has an important role during human embryonic bone and limb development.42 Two patients in our EA/TEF cohort have a duplication in the PAR1 region; the only overlapping duplicated gene is SHOX. The co-occurrence of SHOX duplication in a small cohort of a rare disease such as EA is intriguing, but we cannot exclude a chance finding. Large PAR1 duplications are relatively rare in the database of genomic variation (http://www.dgv.tcag.ca/), a database of ‘healthy' individuals and only two duplications of SHOX are described. In the ISCA consortium patient database (http: http: //www.iscaconsortium.org/), duplications of SHOX are more prevalent (92 in total) and generally classified as uncertain.
However, we observed a SHOX duplication in two EA/TEF patients, and in both these patients limb development is disturbed. The limb and growth anomalies of the thump are different from the wrist deformity usually associated with SHOX deletions and duplications.43, 44 Furthermore, SHOX duplications are also associated with limb anomalies, for example, in Mayer–Rokitansky–Küster–Hauser (MRKH) syndrome type 145, idiopathic short stature46 or Leri–Weill dyschondrosteosis.43
The healthy carriers of the transmitted SHOX duplications in patients 4 and 5 seem to have the exact same duplication/rearrangement as their offspring. Absence of a phenotype in healthy parents could be caused by incomplete penetrance or variable expressivity as described for other microdeletion/duplication syndromes.47 Roos et al48 and Thomas et al49 described five families with inherited SHOX duplications. The duplication was associated with cleft palate in two cases and one patient had a Madelung deformity. These and the SHOX duplications described by Benito-Sanz and co-workers43, 48, 49 are often inherited from an unaffected parent. However, it is important to know the exact location of the SHOX duplication, as insertion of the duplicated segment could result in haploinsufficiency of SHOX by affecting the normal copy of the gene or its regulatory elements. It would certainly be beneficial to determine RNA and protein expression in the esophageal or bone tissue; however, no biopsies of affected and corresponding normal tissues are available at this moment.
The mouse homolog of SHOX (Shox2) is involved in limb development,50 and in humans SHOX enhancers are active in developing limbs.51 Sall1 and Hoxd mutant mice with limb anomalies quite similar to those observed in our patients have overexpression of Shox2.52, 53 SALL1 is mutated in Townes–Brocks syndrome; patients suffering from this syndrome often have anal, renal and thump anomalies, and EA is a variable feature in this syndrome. Sequencing revealed no pathogenic SALL1 mutations in the SHOX duplication patients.
We could speculate that SHOX duplication is the second hit in a two-hit model modulating, not causing, the abnormal development in these patients.
SHOX duplication and other rare inherited copy number variations could be modifying factors contributing to the broad phenotypical spectrum characteristic of the EA/TEF patient population.19
Genetic aberration (eg pathogenic mutations), aneuploidies and structural chromosomal changes like translocations, inversions or copy number variations have previously been detected in ∼12.5% of patients in our cohort. This number will steadily increase, as it is expected that screening previously unresolved cases with whole exome sequencing or improved high-resolution microarray will identify both known and new causal genetic defects. Screening large patient cohorts for genetic defects can delineate new genetic syndromes when genotypes and phenotypes overlap, like recently published for the EFTUD2 gene.54
In conclusion, we describe five patients with sex chromosomal aberrations and EA/TEF. All five patients had duplicated loci of pseudoautosomal genes, including SHOX, that escape X-inactivation and are candidates for a gene dosage effect. As a consequence of the additional X-chromosome, triple X female patients express more transcripts from genes that escape XCI. The expression of one or several of these genes could contribute to the phenotype. Overexpression of XCI escapees could shift the balance from normal to abnormal development in a small percentage of triple X patients. The expression levels of escaping genes on the inactive X-chromosomes may vary between individuals and different tissues.55 As described previously, the phenotypic variability of triple X syndrome ranges widely from subclinical phenotypes to mental retardation and congenital malformations.4
The incidence of triple X syndrome in our EA/TEF cohort is 6.5–11 times higher than expected. Overexpression of XCI escapees, SHOX or other X-linked genes could be responsible for, or modulate, the phenotype of EA/TEF patients.
Acknowledgments
We thank J Hagoort for editorial support. Elisabeth de Jong and Erwin Brosens were funded by the Sophia Foundations for Scientific Research, Projects SSWO-493 and SWOO13-09.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- de Jong EM, Felix JF, de Klein A, Tibboel D. Etiology of esophageal atresia and tracheoesophageal fistula: ‘mind the gap'. Curr Gastroenterol Rep. 2010;12:215–222. doi: 10.1007/s11894-010-0108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw-Smith C. Genetic factors in esophageal atresia, tracheo-esophageal fistula and the VACTERL association: roles for FOXF1 and the 16q24.1 FOX transcription factor gene cluster, and review of the literature. Eur J Med Genet. 2010;53:6–13. doi: 10.1016/j.ejmg.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iskender C, Tarim E, Cok T, Yalcinkaya C, Kalayci H, Sahin F. Fetal axillary cystic hygroma: a novel association with triple X syndrome. Birth Defects Res A. 2012;94:955–957. doi: 10.1002/bdra.23083. [DOI] [PubMed] [Google Scholar]
- Otter M, Schrander-Stumpel CT, Curfs LM. Triple X syndrome: a review of the literature. Eur J Hum Genet. 2010;18:265–271. doi: 10.1038/ejhg.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visootsak J, Graham JM., Jr Klinefelter syndrome and other sex chromosomal aneuploidies. Orphanet J Rare Dis. 2006;1:42. doi: 10.1186/1750-1172-1-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagci S, Muller A, Franz A, et al. Intestinal atresia, encephalocele, and cardiac malformations in infants with 47,XXX: expansion of the phenotypic spectrum and a review of the literature. Fetal Diagn Ther. 2010;27:113–117. doi: 10.1159/000284929. [DOI] [PubMed] [Google Scholar]
- Oddsberg J. Environmental factors in the etiology of esophageal atresia. J Pediatr Gastroenterol Nutr. 2011;52 (Suppl 1:S4–S5. doi: 10.1097/MPG.0b013e3182111c00. [DOI] [PubMed] [Google Scholar]
- Van Opstal D, Boter M, de Jong D, et al. Rapid aneuploidy detection with multiplex ligation-dependent probe amplification: a prospective study of 4000 amniotic fluid samples. Eur J Hum Genet. 2009;17:112–121. doi: 10.1038/ejhg.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Haene B, Hellemans J, Craen M, et al. Improved molecular diagnostics of idiopathic short stature and allied disorders: quantitative polymerase chain reaction-based copy number profiling of SHOX and pseudoautosomal region 1. J Clin Endocrinol Metab. 2010;95:3010–3018. doi: 10.1210/jc.2009-2218. [DOI] [PubMed] [Google Scholar]
- Eussen BH, van de Laar I, Douben H, et al. A familial inverted duplication 2q33–q34 identified and delineated by multiple cytogenetic techniques. Eur J Med Genet. 2007;50:112–119. doi: 10.1016/j.ejmg.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Jonkers I, Barakat TS, Achame EM, et al. RNF12 is an X-Encoded dose-dependent activator of X chromosome inactivation. Cell. 2009;139:999–1011. doi: 10.1016/j.cell.2009.10.034. [DOI] [PubMed] [Google Scholar]
- Jonkers I, Monkhorst K, Rentmeester E, Grootegoed JA, Grosveld F, Gribnau J. Xist RNA is confined to the nuclear territory of the silenced X chromosome throughout the cell cycle. Mol Cell Biol. 2008;28:5583–5594. doi: 10.1128/MCB.02269-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath K, Fang J, Mlynarczyk-Evans SK, et al. Role of histone H3 lysine 27 methylation in X inactivation. Science. 2003;300:131–135. doi: 10.1126/science.1084274. [DOI] [PubMed] [Google Scholar]
- van den Berg IM, Laven JS, Stevens M, et al. X chromosome inactivation is initiated in human preimplantation embryos. Am J Hum Genet. 2009;84:771–779. doi: 10.1016/j.ajhg.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet. 1992;51:1229–1239. [PMC free article] [PubMed] [Google Scholar]
- Gribnau J, Luikenhuis S, Hochedlinger K, Monkhorst K, Jaenisch R. X chromosome choice occurs independently of asynchronous replication timing. J Cell Biol. 2005;168:365–373. doi: 10.1083/jcb.200405117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans AE, Vaarwater J, Paridaens D, et al. Patient survival in uveal melanoma is not affected by oncogenic mutations in GNAQ and GNA11. Br J Cancer. 2013;109:493–496. doi: 10.1038/bjc.2013.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong EM, Felix JF, Deurloo JA, et al. Non-VACTERL-type anomalies are frequent in patients with esophageal atresia/tracheo-esophageal fistula and full or partial VACTERL association. Birth Defects Res A. 2008;82:92–97. doi: 10.1002/bdra.20437. [DOI] [PubMed] [Google Scholar]
- Brosens E, Eussen H, van Bever Y, et al. VACTERL association etiology: the impact of de novo and rare copy number variations. Mol Syndromol. 2013;4:20–26. doi: 10.1159/000345577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon EJ, McEwen GK, Callaway H, Elgar G. Functional analysis of conserved non-coding regions around the short stature hox gene (shox) in whole zebrafish embryos. PLoS One. 2011;6:e21498. doi: 10.1371/journal.pone.0021498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabherwal N, Bangs F, Roth R, et al. Long-range conserved non-coding SHOX sequences regulate expression in developing chicken limb and are associated with short stature phenotypes in human patients. Hum Mol Genet. 2007;16:210–222. doi: 10.1093/hmg/ddl470. [DOI] [PubMed] [Google Scholar]
- Benito-Sanz S, Aza-Carmona M, Rodriguez-Estevez A, et al. Identification of the first PAR1 deletion encompassing upstream SHOX enhancers in a family with idiopathic short stature. Eur J Hum Genet. 2012;20:125–127. doi: 10.1038/ejhg.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer K, Tul N, Nicolaides KH. Maternal serum free beta-hCG and PAPP-A in fetal sex chromosome defects in the first trimester. Prenat Diagn. 2000;20:390–394. doi: 10.1002/(sici)1097-0223(200005)20:5<390::aid-pd824>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Meetei AR, Levitus M, Xue Y, et al. X-linked inheritance of Fanconi anemia complementation group B. Nat Genet. 2004;36:1219–1224. doi: 10.1038/ng1458. [DOI] [PubMed] [Google Scholar]
- Haverty CE, Lin AE, Simpson E, Spence MA, Martin RA.47,XXX associated with malformations Am J Med Genet A 2004125A108–111.author reply 112. [DOI] [PubMed] [Google Scholar]
- Boyd PA, Loane M, Garne E, Khoshnood B, Dolk H. Sex chromosome trisomies in Europe: prevalence, prenatal detection and outcome of pregnancy. Eur J Hum Genet. 2011;19:231–234. doi: 10.1038/ejhg.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guichet A, Briault S, Moraine C, Turleau C, Trisomy X. ACLF (Association des Cytogeneticiens de Langue Francaise) retrospective study. Ann Genet. 1996;39:117–122. [PubMed] [Google Scholar]
- Stewart DA, Netley CT, Park E. Summary of clinical findings of children with 47,XXY, 47,XYY, and 47,XXX karyotypes. Birth Defects Orig Artic Ser. 1982;18:1–5. [PubMed] [Google Scholar]
- Krusinskiene V, Alvesalo L, Sidlauskas A. The craniofacial complex in 47, XXX females. Eur J Orthod. 2005;27:396–401. doi: 10.1093/ejo/cji016. [DOI] [PubMed] [Google Scholar]
- Lin HJ, Ndiforchu F, Patell S. Exstrophy of the cloaca in a 47,XXX child: review of genitourinary malformations in triple-X patients. Am J Med Genet. 1993;45:761–763. doi: 10.1002/ajmg.1320450619. [DOI] [PubMed] [Google Scholar]
- De Veciana M, Major CA, Porto M. Prediction of an abnormal karyotype in fetuses with omphalocele. Prenat Diagn. 1994;14:487–492. doi: 10.1002/pd.1970140613. [DOI] [PubMed] [Google Scholar]
- Hoang MP, Wilson KS, Schneider NR, Timmons CF. Case report of a 22-week fetus with 47,XXX karyotype and multiple lower mesodermal defects. Pediatr Dev Pathol. 1999;2:58–61. doi: 10.1007/s100249900090. [DOI] [PubMed] [Google Scholar]
- Johnston KM, Nevin NC, Park JM. Cloacal defect in a 23-year-old with 47,XXX karyotype and clinical features of Cat Eye syndrome. J Obstet Gynaecol. 2002;22:696. doi: 10.1080/014436102762062457. [DOI] [PubMed] [Google Scholar]
- Trautner MC, Aladangady N, Maalouf E, Misra D. Jejunal atresia in an infant with triple-X syndrome. J Matern Fetal Neonatal Med. 2004;16:198–200. doi: 10.1080/14767050400009147. [DOI] [PubMed] [Google Scholar]
- Rolle U, Linse B, Glasow S, Sandig KR, Richter T, Till H. Duodenal atresia in an infant with triple-X syndrome: a new associated malformation in 47,XXX. Birth Defects Res A. 2007;79:612–613. doi: 10.1002/bdra.20371. [DOI] [PubMed] [Google Scholar]
- Wilkins-Haug L, Porter A, Hawley P, Benson CB. Isolated fetal omphalocele, Beckwith–Wiedemann syndrome, and assisted reproductive technologies. Birth Defects Res A. 2009;85:58–62. doi: 10.1002/bdra.20547. [DOI] [PubMed] [Google Scholar]
- Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- Barakat TS, Jonkers I, Monkhorst K, Gribnau J. X-changing information on X inactivation. Exp Cell Res. 2010;316:679–687. doi: 10.1016/j.yexcr.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Heard E, Disteche CM. Dosage compensation in mammals: fine-tuning the expression of the X chromosome. Genes Dev. 2006;20:1848–1867. doi: 10.1101/gad.1422906. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Greally JM. A stain upon the silence: genes escaping X inactivation. Trends Genet. 2003;19:432–438. doi: 10.1016/S0168-9525(03)00177-X. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Lee NR, Giedd JN. Effects of sex chromosome aneuploidies on brain development: evidence from neuroimaging studies. Dev Disabil Res Rev. 2009;15:318–327. doi: 10.1002/ddrr.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement-Jones M, Schiller S, Rao E, et al. The short stature homeobox gene SHOX is involved in skeletal abnormalities in Turner syndrome. Hum Mol Genet. 2000;9:695–702. doi: 10.1093/hmg/9.5.695. [DOI] [PubMed] [Google Scholar]
- Benito-Sanz S, Barroso E, Heine-Suner D, et al. Clinical and molecular evaluation of SHOX/PAR1 duplications in Leri–Weill dyschondrosteosis (LWD) and idiopathic short stature (ISS) J Clin Endocrinol Metab. 2011;96:E404–E412. doi: 10.1210/jc.2010-1689. [DOI] [PubMed] [Google Scholar]
- Binder G. Short stature due to SHOX deficiency: genotype, phenotype, and therapy. Horm Res Paediatr. 2011;75:81–89. doi: 10.1159/000324105. [DOI] [PubMed] [Google Scholar]
- Gervasini C, Grati FR, Lalatta F, et al. SHOX duplications found in some cases with type I Mayer–Rokitansky–Kuster–Hauser syndrome. Genet Med. 2010;12:634–640. doi: 10.1097/GIM.0b013e3181ed6185. [DOI] [PubMed] [Google Scholar]
- Iughetti L, Capone L, Elsedfy H, et al. Unexpected phenotype in a boy with trisomy of the SHOX gene. J Pediatr Endocrinol Metab. 2010;23:159–169. doi: 10.1515/jpem.2010.23.1-2.159. [DOI] [PubMed] [Google Scholar]
- Ou Z, Berg JS, Yonath H, et al. Microduplications of 22q11.2 are frequently inherited and are associated with variable phenotypes. Genet Med. 2008;10:267–277. doi: 10.1097/GIM.0b013e31816b64c2. [DOI] [PubMed] [Google Scholar]
- Roos L, Brondum Nielsen K, Tumer Z. A duplication encompassing the SHOX gene and the downstream evolutionarily conserved sequences. Am J Med Genet A. 2009;149A:2900–2901. doi: 10.1002/ajmg.a.33118. [DOI] [PubMed] [Google Scholar]
- Thomas NS, Harvey JF, Bunyan DJ, et al. Clinical and molecular characterization of duplications encompassing the human SHOX gene reveal a variable effect on stature. Am J Med Genet A. 2009;149A:1407–1414. doi: 10.1002/ajmg.a.32914. [DOI] [PubMed] [Google Scholar]
- Vickerman L, Neufeld S, Cobb J. Shox2 function couples neural, muscular and skeletal development in the proximal forelimb. Dev Biol. 2011;350:323–336. doi: 10.1016/j.ydbio.2010.11.031. [DOI] [PubMed] [Google Scholar]
- Durand C, Bangs F, Signolet J, Decker E, Tickle C, Rappold G. Enhancer elements upstream of the SHOX gene are active in the developing limb. Eur J Hum Genet. 2010;18:527–532. doi: 10.1038/ejhg.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb J, Duboule D. Comparative analysis of genes downstream of the Hoxd cluster in developing digits and external genitalia. Development. 2005;132:3055–3067. doi: 10.1242/dev.01885. [DOI] [PubMed] [Google Scholar]
- Kiefer SM, Robbins L, Barina A, Zhang Z, Rauchman M. SALL1 truncated protein expression in Townes–Brocks syndrome leads to ectopic expression of downstream genes. Hum Mutat. 2008;29:1133–1140. doi: 10.1002/humu.20759. [DOI] [PubMed] [Google Scholar]
- Gordon CT, Petit F, Oufadem M, et al. EFTUD2 haploinsufficiency leads to syndromic oesophageal atresia. J Med Genet. 2012;49:737–746. doi: 10.1136/jmedgenet-2012-101173. [DOI] [PubMed] [Google Scholar]
- Talebizadeh Z, Simon SD, Butler MG. X chromosome gene expression in human tissues: male and female comparisons. Genomics. 2006;88:675–681. doi: 10.1016/j.ygeno.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood OJ, Hartwell EA, Shattuck KE, Rosenberg HS. Multiple congenital anomalies associated with a 47,XXX chromosome constitution. Am J Med Genet. 1990;36:73–75. doi: 10.1002/ajmg.1320360114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.