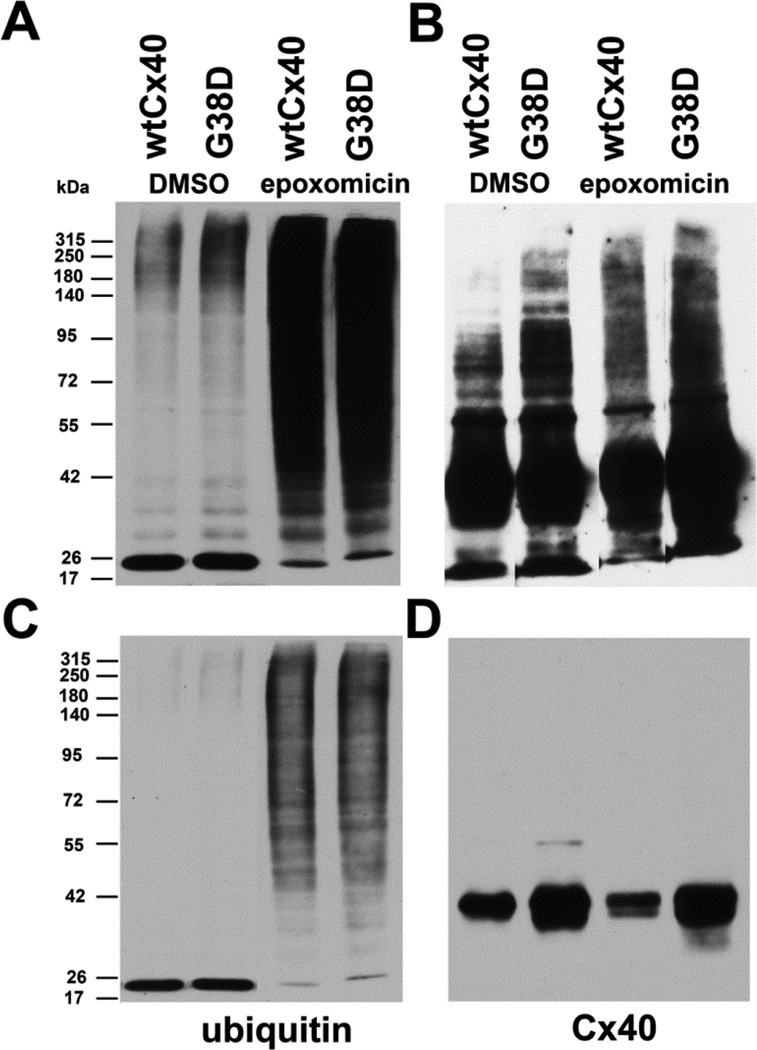
Figure 6. Wild type Cx40 and G38D are ubiquitinated.
HeLa cells stably transfected with wtCx40 or G38D were treated with DMSO (control) or with 0.5µM epoxomicin for 18h. Ubiquitinated proteins were isolated from cell lysates and analyzed by immunoblotting with antibodies directed against ubiquitin (A,C) or Cx40 (B,D). The same blot was probed sequentially with both antibodies with intervening stripping . Eluates equivalent to 10µg of total protein in the starting lysate were loaded in each lane. Top panels (A, B) show long exposures and bottom panels (C,D) show shorter exposures of the same blots. Epoxomicin increased the abundance of ubiquitinated proteins (A,C) in cells expressing either wtCx40 or G38D. Immunoreactive Cx40 was isolated with the ubiquitinated proteins from both cell lines (B,D), and the abundance of slower migrating forms (likely polyubiquitinated Cx40) was increased following epoxomicin treatment (B).