Abstract
Cerebellar research has focused principally on adult motor function. However, the cerebellum also maintains abundant connections with nonmotor brain regions throughout postnatal life. Here we review evidence that the cerebellum may guide the maturation of remote nonmotor neural circuitry and influence cognitive development, with a focus on its relationship with autism. Specific cerebellar zones influence neocortical substrates for social interaction, and we propose that sensitive-period disruption of such internal brain communication can account for autism's key features.
In recent decades, much neuroscience research has focused narrowly on the cerebellum’s role in balance, posture, and motor control. This framework has been explored in the greatest detail in cases where input pathways convey sensory information to the cerebellum, and outputs influence motor effectors. Emerging from this program is the view that the cerebellum acts as a processor that uses a variety of inputs to guide movement.
Receiving much less emphasis has been the role of the cerebellum in higher function. This idea is not new: cognitive roles for cerebellum have been discussed since the mid-19th century (reviewed in Steinlin, 2013), with a resurgence of interest in recent years (D'Angelo and Casali, 2012; Koziol et al., 2014; Mariën et al., 2014). Evidence for cerebellar lesions leading to nonmotor deficits has come from adult cases showing subtle cognitive and affective changes (Stoodley et al., 2012), and congenital cerebellar defects, where deficits are much more pronounced (Basson and Wingate, 2013; Steinlin, 2013).
Two facts have stood in the way of wider recognition of the nonmotor aspects of cerebellar function. First, the most prominent deficits in acute cerebellar injury in adults are of a motor nature. Monitoring the short-term results of injury does not capture long-term consequences that can accumulate over time. The consequences of cerebellar deficit are highly dependent on when the outcome is assessed. Second, cerebellar connectivity is highly differentiated, and focal injury typically leads to focal deficits (Romaniella, 2012). While some cerebellar regions project predominantly to sensorimotor cortex, homologous connections project to cognitive and affective regions, and comprise a large fraction of cerebellar connectivity (Strick et al., 2009). Recently, the extension of this parcellated mapping to nonmotor brain structures has become clearer using modern methods (Buckner et al., 2011; Strick et al., 2009). The cerebellar cortex and nuclei have a distinctive circuit structure that is repeated in a modular fashion throughout the cerebellum, and is highly conserved among vertebrates (Apps and Hawkes, 2009). This has led to the proposal that the cerebellum performs a common algorithm upon a variety of inputs, whether sensory, motor, cognitive, or affective.
In this review, we outline a development-based framework for understanding the nonmotor roles of cerebellum. A variety of observations can be explained by the following unified hypothesis: in addition to its role in the mature brain, the cerebellum acts in early life to shape the function of other brain regions, especially those relating to cognition and affect. We propose that the cerebellum takes an early role in processing external sensory and internally generated information to influence neocortical circuit refinement during developmental sensitive periods. We end by describing how new methods for imaging, mapping, and perturbing neural circuits can be used to explore the complex role of the cerebellum in guiding nonmotor function.
As part of this framework, we propose that cerebellar dysfunction may disrupt the maturation of distant neocortical circuits. To summarize the concept of developmental influence between brain regions, we use the term developmental diaschisis. Diaschisis (\dī-as'-kə-səs\; Gr. dia: across, schisis: break) is an existing neurological term indicating a sharp inhibition in activity at a site that is distant from a site of injury, but is anatomically connected with it through fiber tracts. For example, prefrontal injury has been shown to lead to abrupt decreases in blood flow to the contralateral cerebellum, and vice versa. In the same way, we define developmental diaschisis as a phenomenon in which disruptions in activity in a particular brain area, such as the cerebellum, can affect the organization and function of other, remote brain sites over developmental time. As a central example, we will focus on autism spectrum disorder (ASD), for which the developmental-diaschisis hypothesis can resolve some longstanding puzzles regarding the cerebellum’s role.
Autism-related gene co-expression identifies cerebellar and neocortical sites of disruption
Autism spectrum disorder (ASD), one of the most strongly heritable major neurodevelopmental disorders (Abrahams and Geschwind, 2008), has attracted tremendous research interest. Usually diagnosable by the age of 2 (http://cdc.gov/ncbddd/autism/data.html and reviewed in Daniels et al., 2014), ASD is highly heterogeneous, and encompasses a wide range of deficits including social impairment, communication difficulties, and repetitive and stereotyped behaviors. A Web Of Science literature search reveals over 34,000 scientific publications mentioning autism since Kanner’s original description (Kanner, 1943), more than half of which have been published since 2008.
Generally speaking, fetal brain development is driven by a genetic program that can be driven off track by genetic or environmental perturbations. One theme emerging from the considerable autism research literature is the idea that fetal brain development can be perturbed by any of hundreds of autism risk alleles (SFARI GENE; https://gene.sfari.org). Inherited genetic variation accounts for ~40% of the risk for ASD (Stein et al., 2013), with each allele contributing a small fraction to the total risk. In most cases, each allele is a variant of an essential gene, and its presence most often leads to normal-range function (Leblond et al., 2012; O'Roak et al., 2011). Thus most autistic children have two neurotypical parents. First-degree relatives of persons with ASD often show distinctive mental traits, including unusual social and emotional characteristics (Sasson et al., 2013) and an interest in technical subjects (Baron-Cohen et al., 1998; Campbell and Wang, 2012), indicating that ASD risk genes may drive variations of outcome within the normal range. In this sense development is robust, and combinations of genes are likely to work together to trigger ASD. However, despite this booming literature, it is not yet established how genetic risks drive specific missteps in the maturation of brain circuitry.
Three recent computational studies have used aggregated gene expression patterns to ask when and where ASD genes are expressed (Figure 1a; Menashe et al., 2013; Parikshak et al., 2013; Willsey et al., 2013). Some ASD susceptibility genes show a high degree of co-expression with one another in mouse and human brain, allowing the identification of specific gene networks or “cliques” (Menashe et al., 2013). ASD-related co-expression networks have been found during two distinct periods of development. First, during human gestational weeks 10–24 and mouse P0–10, expression occurs in a broadly defined somato-motor-frontal region (Willsey et al., 2013) especially in layer 5/6 cortical projection neurons (Parikshak et al., 2013; Willsey et al., 2013) and other layers (Parikshak et al., 2013). Second, in humans from neonatal to age 6, cerebellar network expression is strong (Willsey et al., 2013), particularly in the cerebellar granule cell layer (Menashe et al., 2013). Unfortunately, the third recent study examining aggregated gene co-expression patterns did not examine cerebellum (Parikshak et al., 2013).
Figure 1. The cerebellum as a mediator of ASD risk.
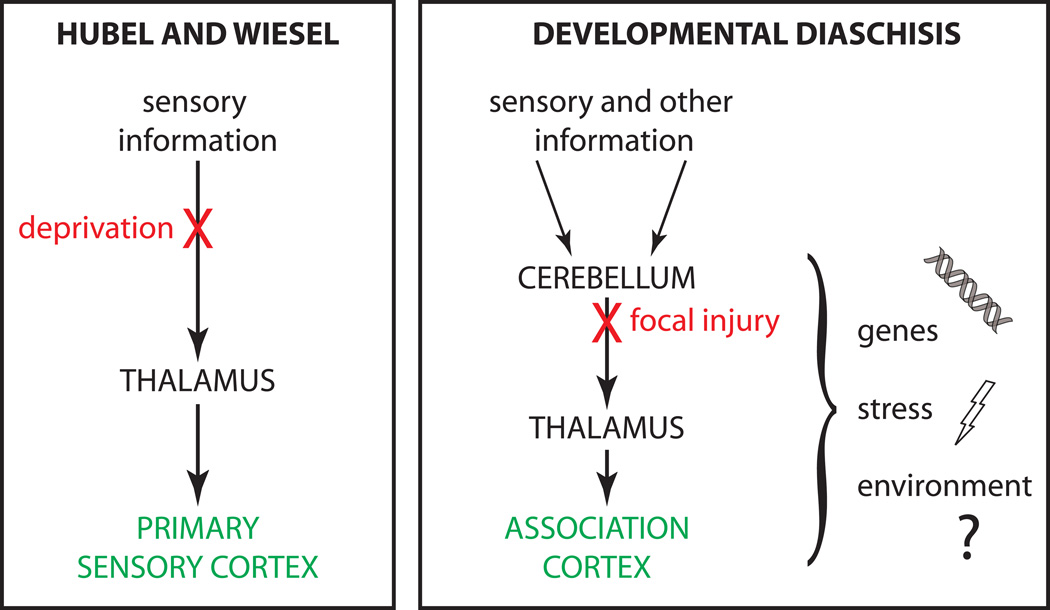
(a) Patterns of ASD gene co-expression show specific expression in cerebellum during early postnatal years (image adapted from (Menashe et al., 2013); see also (Willsey et al., 2013). (b) Risk ratios for ASD for a variety of probable genetic (light blue) and environmental (dark blue) factors. Risk ratios were taken directly from the literature except for the largest four risks, which were calculated relative to the US general-population risk. At 36X, cerebellar injury carries the largest single non-heritable risk. For explanation of other risks see text.
Taken together, these patterns identify two regions where genetically driven ASD-related developmental programs can go off track: the second-trimester frontal/somatomotor neocortex and the perinatal/postnatal cerebellar cortex. Based on gene ontology classification, many of the co-expressed ASD susceptibility genes are involved in synaptic plasticity, development, and neuronal differentiation (Parikshak et al., 2013), indicating disruptions in neural circuit formation and plasticity as targets for investigation.
Perinatal risks for autism highlight a role for the cerebellum
The diverse body of autism research provides an opportunity to quantify the contribution of a wide range of risks, with the goal of identifying putative neural substrates and mechanisms. Just as there are two major periods of ASD gene co-expression, epidemiological and clinical literature reveal two major time windows for environmental risk. These time windows, identified independently from the gene expression analysis, suggest a postnatal period when the cerebellum might influence ASD-like outcomes.
To illustrate both genetic and environmental risk factors for autism in quantitative perspective, a variety of associated risk ratios are shown in Figure 1b. The highest risk ratio is found for identical twins with a substantially lower risk for fraternal twins, a finding that formed the original basis for the idea of genetic causation. Yet ASD is also affected by environmental factors occurring before birth, demonstrating the potential of environmental risk factors to impede the maturation of social function.
A large body of research has investigated the hypothesis that the developing brain may be particularly vulnerable to maternal stress and other environmental insults before, at, and after birth (McEwen, 2007; Kinney et al., 2008b). The effects of maternal infection during pregnancy, especially the second and third trimester (Atladottir et al., 2010), suggest that activation of the maternal stress response, including glucocorticoid signaling and the immune response (Patterson, 2012), may disrupt brain development. Experimentally, brain development in rodent pups, which closely resembles brain development in humans during the second and third semester (Workman et al., 2013), is influenced by stress due to variation in maternal care, leading to epigenetic variation (Gudsnuk and Champagne, 2011) and long-term changes in behavior (Moriceau et al., 2010). Stress in rodent pups alters the excitable properties of CNS neurons (Schneider et al., 2013), decreases hypothalamic-pituitary-gonadal axis reactivity (McEwen, 2007), and impairs cerebellar learning in adulthood (Wilber et al., 2011). Speculatively, in the case of ASD such mechanisms might underlie the effects of premature birth (Moster et al., 2008), elective cesarean section (Glasson et al., 2004), being born to mothers caught in a hurricane strike zone (Kinney et al., 2008a), maternal emigration (Magnusson et al., 2012), and maternal post-traumatic stress disorder (Roberts, 2014), all of which have been shown to be positively correlated with risk for autism in the offspring. All of these autism risks are larger than the risk associated with advanced maternal or paternal age, and suggest a period of stress-sensitivity that starts before birth.
All of the nongenetic ASD factors shown are associated with risk ratios between 2 and 7, with one notable exception: injury to the cerebellum. Early disruption of the cerebellar circuitry has been shown to be positively correlated with autism (Beversdorf et al., 2005; Courchesne et al., 2001; Hashimoto et al., 1995; Limperopoulos et al., 2007). Damage to the cerebellum at birth (Limperopoulos et al., 2007) leads to high scores on the M-CHAT and Vineland autism screening inventories with a risk ratio as high as 40. These studies suggest that cerebellar insult is a very strong risk factor for ASD, affecting a wide range of cognition and warranting follow-up using rigorous diagnostic methods (Chlebowski et al., 2013). The risk ratio is at the high end for exogenous risks, and is comparable to that of genome-wide twin risk and to the highest-risk single mutations for autism. As a point of quantitative comparison, cigarette smoking increases the risk of lung cancer by a factor of 20 to 40 (Pope et al., 2011). These findings suggest that after birth, the cerebellum plays an essential role in the development of basic social capabilities. This idea is consistent with the fact that the cerebellum is among the most frequently disrupted brain regions in autistic patients, at both microscopic and gross levels (Courchesne et al., 2005; Palmen et al., 2004). Indeed, cerebellar defects in ASD are seen throughout life, and if they arise by birth are often sufficient to cause the disorder (as reviewed in detail in the next section).
A second time window of vulnerability to ASD occurs in the postnatal years and suggests a role for experience. Autism becomes apparent during early childhood, usually in what developmental psychologists define as the sensorimotor stage of development (Piaget, 1983). Social and/or sensory deprivation during early childhood can also lead to autism-like social deficits in adulthood. In a study of children adopted from abusive Romanian orphanages into UK families, a high fraction of children who underwent long-term deprivation developed social deficits that closely resembled autism, which could be reversed by placement in a normal adoptive home (Rutter et al., 1999; Smyke et al., 2009). The longer and later the children stayed in deprived conditions, the more severe and difficult the behavioral changes were to reverse. Thus experience-dependent mechanisms are likely to guide the formation of social capacities during the critical first years of life.
These identified risks are likely to share at least some common mechanisms. Genetic risks and epidemiologically identified environmental factors most likely act by influencing the developmental program of the nervous system. These risk factors are triggers that act upon as-yet-unidentified neural substrates. In this context, is early-life brain injury a general ASD risk factor, or is the cerebellum a special point of vulnerability?
Are autism-like outcomes from early injury specific to the cerebellum?
Because ASD arises early in development and eventually involves multiple brain structures, focal brain injury studies in early postnatal life can provide valuable information about how ASD unfolds. Although focal brain injury is not thought to be a principal cause of developmental disorders, it provides an approach to systems-level perturbation that deepens the significance of gene expression studies. Here we present focal perturbation data to identify candidate subsystems that may drive the maturation of brain capacities.
Of particular interest for ASD are sites at which early-life injury, but not adult injury, leads to a long-term deficit; we call these developmental upstream drivers. We call sites at which adult injury leads to long-term ASD-like deficits downstream targets (Figure 2). A classical example of an upstream driver is the role of retina and thalamus in shaping the circuitry of a downstream target, the primary visual cortex. In this example and others, early-life deprivation during a sensitive period can lead to commitments that are difficult to reverse at later ages. More complex functions tend to have sensitive periods that come even later during development (Knudsen, 2004), so the primary visual cortex is itself an upstream driver in the later maturation of yet more complex visual functions.
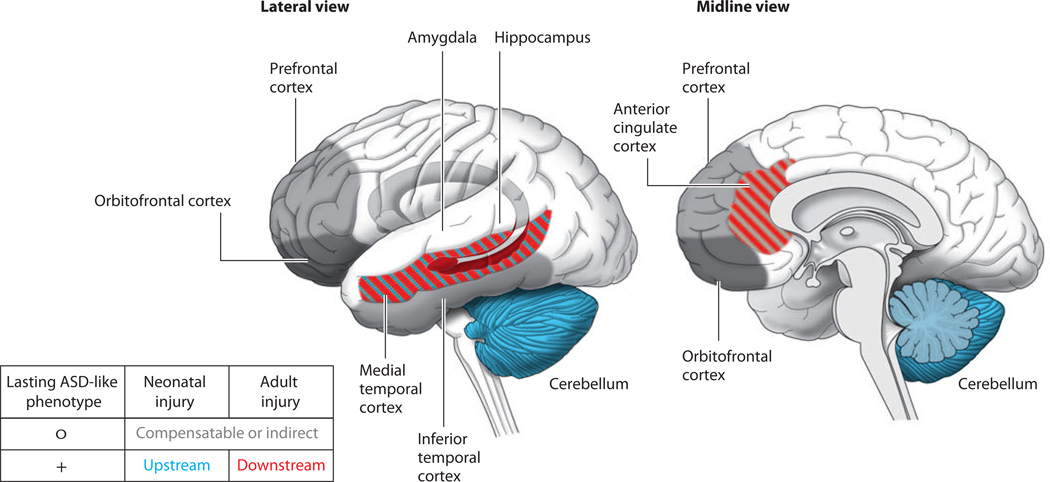
Figure 2. A developmental diaschisis model for neurodevelopmental disorders.
(left) A diagram of activity-dependent influences on neural circuit refinement in primary sensory neocortex during sensitive periods of development, as articulated by Hubel and Wiesel. (right) A proposed generalization for the influence of cerebellar processing of multisensory information on neocortical areas essential for social and cognitive processing.
In this classification scheme, many brain regions would be expected to fall into the downstream category for ASD, since cognitive and social functions engage neural substrates throughout the brain. A third category, which we call compensatable, encompasses brain regions in which an acute injury’s effects diminish over time due to plasticity mechanisms for recovering function.
We will now apply this downstream/upstream/compensatable framework to ASD-like social outcomes. We focus on the core deficits of autism: impaired social interaction and emotional reciprocity, impaired verbal and non-verbal communication, restricted interests, and repetitive or stereotyped actions or thoughts. These symptoms are distinct from “higher” social deficits in which dysfunctions require the ability to react to a social situation in the first place: Examples include persistent increased irritability (e.g. orbitofrontal syndrome; Chow, 2000), difficulties maintaining friendships (e.g. damage to the prefrontal cortex; Eslinger et al., 2004), social fear or anxiety (e.g. generalized anxiety disorder; De Bellis, 2000) and inappropriateness of social interaction (e.g. Williams Syndrome; Meyer-Linderberg et al., 2005). We will consider a number of brain areas that have known probable roles in supporting cognition and affect (Figure 3). The general principle emerging from these studies is that with the exception of the cerebellum, ASD-like deficits arising from early-life lesions are to a large degree recoverable over time.
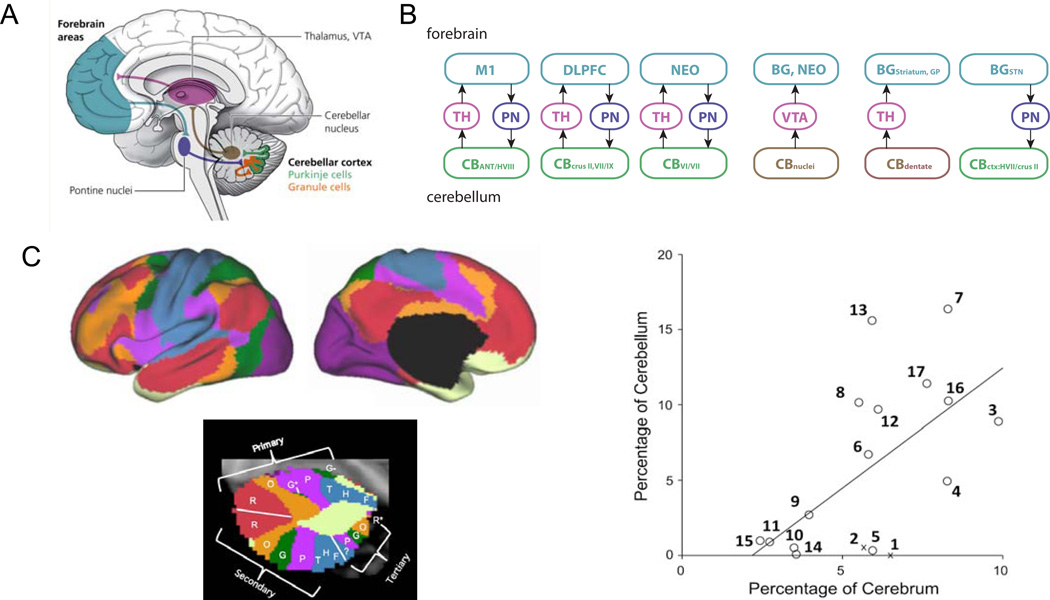
Figure 3. Upstream influences and downstream targets in autism spectrum disorder (ASD).
Categorization of regions showing abnormalities in ASD brains according to whether they result in lasting ASD-like social deficits when injured neonatally (upstream), result in lasting ASD-like social deficits when injured in adulthood (downstream), or can be fully or partially compensated after an injury, regardless of age (compensatable).
Amygdala, hippocampus, and the medial temporal lobe
The importance of the amygdala in emotional response triggered considerable initial interest in investigations of ASD (Baron-Cohen et al., 2000). As a test of the amygdala’s involvement as an upstream cause of ASD dysfunctions, ibotenic acid injections have been done in macaque monkeys to specifically lesion cells while sparing fibers of passage (reviewed by Bliss-Moreau et al., 2011). Using this method, early damage to the amygdala does not alter fundamental features of social development, including the development of mother-infant interactions and the ability to interact with peers. Selective deficits eventually appear, including stereotypies, blunted processing (with recognition intact) of emotionally evocative video stimuli, and reduced social fear. In humans, complete congenital absence of the amygdala on both sides leads to relatively mild social deficits and low scores on standardized ASD inventories (Paul et al., 2010). Adult amygdala lesion in macaques also leads to decreased anxiety and social fear and increased social confidence. In short, although the amygdala is important in affective processing, it is not needed for the capacity to identify socially meaningful contexts. Nonetheless, both anatomical and functional abnormalities in the amygdaloid complex are observed in a variety of neuropsychiatric disorders (Schumann et al., 2011), including ASD. This pattern of evidence suggests that a dysfunctional amygdala may be a downstream target in the etiology of ASD, with a possible upstream role in the case of stereotypies.
Broader lesions reveal that when damage encompasses neighbors of the amygdala, more profound symptoms emerge (Bliss-Moreau et al., 2011; Machado and Bachevalier, 2006). Adult and neonatal lesions to the hippocampus, which has close connections to other parts of the MTL, lead to only limited social defects (Bliss-Moreau et al., 2011). However, combined lesion in 2-week-old macaques of amygdala, hippocampus, and the overlying medial temporal cortex, a structure implicated in Klüver-Bucy syndrome, leads to severe social symptoms by 6 months of age and persisting into adulthood. These symptoms include failure to initiate social contacts, failure to accept social approaches by peers, and failure to make eye contact. Neonatal lesions to MTL produce more severe core social deficits than adult lesions, indicating that MTL structures as a whole may act in a developmentally upstream fashion in the emergence of social capacities. Also, these findings do not rule out an upstream role for the medial temporal cortex acting by itself.
Inferior temporal cortex
One structure related to the medial temporal structures is the inferior temporal cortex, which is involved in face recognition. Adult lesion of this structure leads to face blindness and behavioral abnormalities such as hyperorality and decreased aggression, but if the lesion is done neonatally, these signs fade considerably over time (Málková et al., 2010). Thus inferior temporal cortex, which is well studied in ASD patients, might be regarded as a structure whose contributions to core social capacity are compensatable.
Frontal neocortical regions, including the anterior cingulate cortex
Disruptions to the structure of frontal neocortex have been reported in ASD (Courchesne et al., 2011; Girgis et al., 2007; Stoner et al., 2014). Perturbation of prefrontal and orbitofrontal cortex do not lead to ASD-like symptoms. In the orbitofrontal cortex (OFC), which is heavily connected to the amygdala, both neonatal and adult damage in humans and in animals impair the regulation of emotions in social situations and emotion-based decision-making, and responsiveness to changing social and behavioral environments, but no disability in basic social interactions (Bachevalier and Loveland, 2006; Bachevalier et al., 2011; Machado and Bachevalier, 2006). Although neonatal damage results in fewer initiated social interactions in nonhuman primates, it remains unknown whether this deficit persists into adulthood; other studies of OFC damage suggest that adult damage leads to more severe cognitive consequences than neonatal damage (Bachevalier and Loveland, 2006). A similar case may occur with the prefrontal cortex, in which perinatal damage leads to persistent increased irritability, difficulties maintaining friendships, and lack of empathy and fear, but ASD-like social dysfunction is absent (Eslinger et al., 2004).
One region linked to more serious deficits in basic affective interaction is the anterior cingulate cortex (ACC), which is strongly connected with amygdala and OFC. Adult lesion to the ACC in non-human primates produces lack of interest in social situations, loss of emotional regulation, and inability to recognize social and emotional cues (Devinsky et al., 1995; Hadland et al., 2003). Thus the ACC is necessary in the mature brain to carry out core social functions and is therefore not an upstream structure. Because neonatal lesions of ACC have not been reported, at the time of this writing it is unresolved whether its contribution is downstream, or if it is compensatable by other brain regions.
Cerebellum
In adults, cerebellar lesions are unlikely to result in profound social deficits. However, damage can produce cerebellar cognitive-affective syndrome, which is characterized by disturbances of planning, decision-making and working memory, deficits in visuo-spatial reasoning, speech-generation deficits, verbal reasoning defects, personality changes, anxiety, and blunted or inappropriate social behavior (Koziol et al., 2014; Schmahmann, 2004; Wolf et al., 2009). This syndrome is predominantly reported after injury to the posterior cerebellum, with cognitive symptoms associated with the cerebellar hemispheres and affective symptoms with the vermis (Stoodley et al., 2012).
Lesions at earlier ages lead to more conspicuous cognitive and affective changes, and the nature of the developmental delay depends on the area that is injured. Damage to the hemispheres results in language delay and visual and verbal reasoning deficits, and damage to the vermis results in withdrawn social behavior, impaired gaze, anxiety, and stereotyped behavior (Wells et al., 2008). In children ages 6 to 13, posterior fossa damage, particularly to the posterior vermis, has been known to produce cerebellar mutism, a syndrome in which language capacities regress by years, sometimes leading to total loss of the power of speech (Riva and Giorgi, 2000). Language deficits appear to go beyond purely problems of phonological speech production, as they involve specific loss of grammar and/or vocabulary. Mutism is often not permanent, indicating the existence of compensatory mechanisms elsewhere in the brain.
This trend toward cognitive and affective deficits is particularly striking when cerebellar damage occurs near the time of birth. Perinatal damage to the cerebellum due to premature birth or as a secondary consequence of surgery produced social deficits and high scores on ASD inventories at a rate of 37–59% (Bolduc et al., 2012; Limperopoulos et al., 2007). Hypoplasia of the posterior cerebellar vermis strongly predicted autism evaluation scores (Bolduc et al., 2012), and perinatal cerebellar damage led to a relative reduction in volume of the contralateral prefrontal cortex at age two (Bolduc et al., 2012; Limperopoulos et al., 2014). In addition, a number of cerebellar malformation syndromes have ASD-like signs, including Joubert syndrome, Dandy–Walker malformation, and pontocerebellar hypoplasia, all of which often include substantial delays in intellectual, cognitive, and social function in cases where the vermis is malformed (Boltshauser, 2004). In Joubert syndrome, 25% of cases are diagnosed with ASD (Geschwind and Levitt, 2007). These findings indicate that neonatal damage to the cerebellum can have persistent structural and functional consequences.
Structural analysis from live imaging and postmortem studies shows that in ASD, cerebellar abnormalities are present in early life and persist until adulthood (Abell et al., 1999; Becker and Stoodley, 2013; Wegiel et al., 2010). Persistent cerebellar volume differences emerge starting in the first two years of life (Hashimoto et al., 1995; Stanfield et al., 2008). Patients as young as 2–3 years old show vermal hypoplasia but an increase in white matter relative to gray matter in the cerebellar hemispheres (Courchesne et al., 2001; Courchesne et al., 2011). Cerebellar undergrowth—particularly in the vermis—has been associated with increases in frontal volume in 3–9 year-old ASD boys (Carper and Courchesne, 2000; Sparks et al., 2002). Meta-analyses of structural imaging studies have shown cross-sectional area decreases in the posterior vermis (lobules VI–VII) across ASD patients, particularly in children under the age of 10 (Courchesne, 2011; Stanfield et al., 2008). In addition, a subregion of vermis shows hypoplasia in adults diagnosed with infantile autism (Courchesne et al., 1988; Kaufmann et al., 2003; Scott et al., 2009). Some of these abnormalities decline or reverse at later ages (Courchesne et al., 2011). Gross cerebellar abnormalities are matched by cellular abnormalities in Purkinje cells and the cerebellum’s major input and output structures, the inferior olive and the deep nuclei (Palmen et al., 2004), differences which are apparent by age four (Bailey et al., 1998). In summary, starting from the earliest ages when core ASD deficits appear, the cerebellum shows both gross and cellular defects, especially in the vermis.
Importantly, effects of early-life cerebellar damage on cognitive and affective function have been successfully modeled in animal studies (Becker and Stoodley, 2013). In rats, midline (i.e. vermis) cerebellar lesions in pups and juveniles result in later perseveration abnormalities in social behavior and vocalization (Al-Afif et al., 2013; Bobée et al., 2000). A recent genetically based example comes from a mouse model of tuberous sclerosis, which in humans shows cerebellar pathology and a 25% rate of autism diagnosis (Smalley, 1998). Purkinje cell-specific knockout of the tuberous sclerosis gene TSC1 leads to autism-like deficits, including deficient social interaction with other mice, increased repetitive self-grooming, diminished mother-pup interaction, and perseveration when the rule is switched on a T-maze (Tsai et al., 2012). These deficits were rescued by the administration of rapamycin (a drug that epistatically rescues the TSC1 deletion) at postnatal day 7. The TSC1 study is an example of experimentally induced developmental diaschisis, and provides an important demonstration that early-life cerebellar development can play a necessary role in acquiring core social capacities.
The demonstration that cerebellum-specific insult is sufficient to generate ASD-like symptoms brings added meaning to global transgenics, in which all cells are affected. Cerebellar disruption and behavioral abnormality occur together in a number of mouse ASD-related models, including CAPS2 (Sadakata et al., 2007; Sadakata et al., 2012), Engrailed-2 (Brielmaier et al., 2012), Fragile X (Koekkoek et al., 2005), Mecp2 (Ben-Shachar et al., 2009), and neuroligin-3 (Baudouin et al., 2012). In addition, region-specific differences in cerebellar structure have been found in five autism mouse models (Ellegood et al., 2010; Steadman et al., 2013; Ellegood et al., 2014). A broader bioinformatic examination of mouse models suggests that linkages between cerebellum and ASD may be fairly general: in an analysis of gene-phenotype associations (Meehan et al., 2011), ASD-related genes were found to be associated with a group of phenotypes that included not only social defects, but also abnormal motor behavior and cerebellar foliation. These results collectively open the possibility that in a broad array of ASD mouse models, cognitive dysfunction may arise in part from cerebellum-specific dysfunction.
The cerebellum can influence cognitive and affective-related forebrain structures via long-distance loops
The cerebellum occupies a relatively constant fraction of the mammalian brain, independent of the proportions of other components (Clark et al., 2001). Since the cerebellum is connected with many brain regions, its role in integrative brain function is likely to be general and similar across species. The cerebellum’s circuit architecture repeats nearly identically throughout its extent. Thus it may execute a single canonical circuit computation – but with functional consequences that will vary depending on where it sends its output and on the stage of development.
In the case of neocortex, a general organizational principle is that of cerebello-thalamo-cortical loops (Figure 4). Cerebello-thalamo-cortical loops (Figure 4a,b) have long been appreciated for motor functions (Prevosto et al., 2010; Strick et al., 2009; Voogd et al., 2012). This loop organization also encompasses brain regions known to contribute to cognitive and affective processing (Strick et al., 2009). For example, transsynaptic viral tracing in monkeys and electrical stimulation in rats (Watson et al., 2009) reveals a bidirectional loop joining the dorsolateral prefrontal cortex with lateral crus II and vermal lobules VII on the contralateral side. An even broader picture of the map between neocortex and cerebellum comes from human resting-state functional imaging measurements of covariation between neocortex and cerebellum (Figure 4c). These measurements reveal that nearly every part of neocortex has a cognate region in the cerebellum (Buckner et al., 2011; Krienen and Buckner, 2009). The representations are approximately proportional, so that larger functional areas in the neocortical sheet have larger partners in cerebellum (Figure 4c). Notably, cerebellar regions associated with autism communicate with frontal regions of neocortex. Prefrontal cortex is associated with the posterior cerebellar hemispheres (Krienen and Buckner, 2009), and lobules VI and VII of the vermis are associated with mid-frontal regions that appear to encompass ACC in humans (Buckner et al., 2011) and homologous regions in rats (Galgliani, 2012; Suzuki et al., 2012).
Figure 4. The cerebellum and forebrain are bidirectionally linked in an orderly mapping.
(a) The general structure of cerebello-thalamo-cortical loops. Each projection indicates a monosynaptic pathway. The pontine-cerebellar and deep nuclear-thalamic projections cross the midline to the contralateral side. (b) Regions are mapped precisely to form closed loops as demonstrated using classical and viral tracing methods in rodents and nonhuman primates. Adapted from (Strick et al., 2009). Loop-specific connectivity through the thalamus and pons connects the anterior cerebellum, cerebellar crus II/lobules VII-IX, and cerebellar lobules VI/VII with motor cortex (M1), dorsolateral prefrontal cortex (DLPFC) (Kelly and Strick, 2003), and areas of the neocortex (NEO) (Suzuki et al., 2012), respectively. An ascending pathway projects monosynaptically from the cerebellar nuclei to the ventral tegmental area (VTA) (Snider and Maiti, 1976). A descending pathway joins the subthalamic nuclei (STN) with cerebellar-cortical hemispheric lobule VII and crus II while an ascending pathway joins the cerebellar nuclei with the striatum and globus pallidus (Hoshi et al., 2005; Bostan and Strick, 2010). (c) In human brains, spontaneous waking activity measured using functional magnetic resonance imaging reveals a parcellated relationship of covarying activity between corresponding zones of cerebellum and neocortex. The olored maps at left indicate 7 zones in which a single color denotes regions of neocortex and cerebellar cortex with strongly covarying activity. The plot at right indicates the fraction of neocortex in each zone of a 17-zone map, plotted against the fraction of cerebellar cortex in the corresponding zone. This plot indicates that representation in the neocortical and cerebellar cortical sheets is approximately proportional (Buckner et al., 2011).
As another example of cognitive/affective related connectivity, the cerebellar nuclei project to parts of the basal ganglia associated with reward. The cerebellum sends indirect connections to basal ganglia via the thalamus (Hoshi et al., 2005). In addition, a long line of evidence using both transported tracers (Phillipson, 1979; Geisler and Zahm, 2005) and viruses (Watabe-Uchida et al., 2012) shows that the deep nuclei project monosynaptically to the ventral tegmental area, a structure central to the signaling of reward (Schultz, 2002). In the descending direction, the basal ganglia send a pathway from the subthalamic nucleus back to the contralateral cerebellar hemisphere (Bostan and Strick, 2010). Thus long-distance cerebellar loops may participate in shaping dopamine-based reward and other unexpected events that influence reinforcement learning.
Several locations in cerebello-thalamo-cortical loops are potential targets of ASD-related gene expression. As previously stated, the cerebellum is a site of co-expression in early postnatal years, especially in granule cells (Menashe et al., 2013; Willsey et al., 2013). A second site for ASD gene co-expression is deep-layer projection neurons of the neocortex during the second trimester (Willsey et al., 2013). Corticopontine projections originate from layer 5, suggesting that this arm of the cerebello-thalamo-cortical loop may be vulnerable to ASD genetic risks. Therefore the concept of developmental diaschisis extends beyond focal lesion, and can include the possibility that disrupted molecular signaling pathways can interrupt long-distance guidance of neural circuit refinement.
Do cerebellar brain pathways drive sensitive-period maturation of associative neocortex?
The foregoing findings point to a role during early life for the cerebellum to shape the eventual organization of mature brain functions. The consequences of early-life damage to the cerebellum are similar to the effects of social deprivation. Both abnormal processing within the brain and deprivation of external social input could disrupt the maturation of downstream circuits in a similar fashion.
Strikingly, the cognitive and social consequences of cerebellar injury show an opposite age-dependence from the motor consequences. When motor-related cerebellar regions are lesioned, adult injury leads to ataxia, dysarthria, dysphagia, and other problems of muscular coordination and timing (Timmann et al., 2008). These motor dysfunctions attenuate with time. In children, acquired lesions of the cerebellar hemisphere lead to motor development that is normal or only moderately delayed (Tavano et al., 2007). In very preterm children, no correlation has been observed between the volume of the underdeveloped cerebellum and motor function later in childhood (Allin et al., 2001). Long-term compensation is unlikely only in cerebellar agenesis, in which motor function remains underdeveloped throughout life (Timmann et al., 2003). Thus, the cerebellum is compensatable with respect to motor functions, but cognitive and social functions are specifically vulnerable to early-life perturbation of cerebellum – suggesting a sensitive-period mechanism.
Normal experience is an essential component of brain development (Figure 2). Activity in one brain area can induce nearly irreversible changes in another brain area through structural and synaptic plasticity mechanisms (Hensch, 2005). Long-range projections are sculpted by local circuit dynamics, the best-known case being that of thalamocortical systems. In primary visual cortex, mismatch of visual input due to monocular deprivation during the sensitive period for ocular dominance column formation leads to an overrepresentation of the nondeprived eye and a failure to create a binocular map. Similarly, a sensitive period has been observed for auditory processing in mice (Barkat et al., 2011; Yang et al., 2012). Overall, in the process of experience-expectant plasticity, developing brains go through sensitive periods (Knudsen, 2004; Wiesel, 1981) during which they require a minimum level of normal experience. In light of these findings, cerebellar-thalamic-neocortical communication may also shape the refinement of neocortical circuitry.
Dendritic and axonal mechanisms for sensitive-period refinement
A characteristic pattern of cellular growth during sensitive periods is initial exuberant growth of dendritic and axonal arborizations, followed by activity-dependent pruning of unwanted connections. These phenomena have been observed in diverse systems that include mammals, songbirds, and barn owls (reviewed in Knudsen, 2004). In activity-dependent plasticity, incoming information is a driver of circuit refinement. The same pattern may apply to developmental disorders. For example, cerebral palsy can be modeled in animals by blocking corticospinal activity on one side in early postnatal life (Friel and Martin, 2007). In this case, descending corticospinal projections normally undergo an initial period of bilateral mapping, after which Hebbian activity-dependent competition leads to the preferential elimination of ipsilateral projections. Blockade of activity prevents this process and the functional separation of the two tracts does not occur.
Failures of neocortical pruning might be expected to have volumetric correlates. In infants who later go on to develop autism, increased net brain growth is apparent by age 1, as quantified by increased head circumference (Stigler et al., 2011). Extreme head growth is associated with the most severe clinical signs of autism (Courchesne et al., 2004). In volumetric MRI measurements, ASD brains grow faster on average than neurotypical brains in the first two postnatal years (Redcay and Courchesne, 2005). By age 2.5, brain overgrowth is visible as enlargement of neocortical gray and white matter in frontal, temporal, and cingulate cortex (Schumann et al., 2010). Since this abnormal growth comes after the time of neurogenesis, volume differences are likely to arise either from disruption of progressive (growth) or regressive (pruning) events. Disruption to either of these processes could account for perturbations in the trajectory of gross volume changes. Additional contributions could also come from changes in glial volume or number (Schumann and Nordahl, 2011). Finally, overgrowth in ASD brains is followed by premature arrest of brain growth after age 4. These abnormalities would be expected from defects in plasticity mechanisms – for example, dendritic growth and pruning, or axonal branching.
Such a deficit in sensitive-period circuit refinement could arise in two ways. First, inappropriate input, as originally described by Hubel and Wiesel, could fail to instruct developing circuitry through Hebbian plasticity mechanisms. This could occur if subcortical structures, including the cerebellum, were perturbed. For example, reduced numbers of Purkinje cells, which are inhibitory, could allow abnormally high levels of firing by deep-nuclear projection neurons. Second, plasticity mechanisms themselves could be perturbed by specific alleles of the genes that govern those mechanisms. Both cases amount to a failure of postnatal experience to have its normal effects on the neocortex. Such a failure could contribute to the blunting of regional differences in gene expression across neocortical regions that is seen in autistic subjects (Voineagu et al., 2011).
Sensitive periods for cognitive and social function
Higher sensory capabilities are thought to undergo sensitive periods once lower sensory structures have matured (Knudsen, 2004). A similar principle is likely to apply to cognitive functions. One candidate example is the ontogeny of reading (Turkeltaub et al., 2003). In early readers, activated brain regions are distributed on both sides of the neocortex and cerebellum. Between childhood and adolescence, these regions come to exclude auditory regions, leaving a more focused, largely left-hemisphere network that includes the visual word form area. Notably, in readers who first learn to read as adults, activity patterns are more bilaterally distributed (Dehaene et al., 2010) and are reminiscent of literate children starting to read, indicating that adult circuitry has considerably less capacity for refinement.
The sensitive-period concept also applies to social development. In social isolation experiments by Harry Harlow and others in the 1960s, infant monkeys were raised under conditions in which the birth mothers were replaced with artificial surrogates for the first six months of their lives. At later ages, these deprived monkeys displayed rocking behavior, perseveration, and inability to communicate or socially bond with other monkeys (Novak and Suomi, 2008). This work supported a critical period hypothesis for social function, and it was later found that targeted interventions using peer monkeys could partially rescue the effects on previously isolated monkeys.
Like the plight of the Romanian orphanage children, the Harlow experiments are disturbing because the degree of deprivation is extreme. The developmental diaschisis hypothesis raises the possibility that ASD has similarly profound effects on forebrain circuit maturation. The difference is that the flow of information is interrupted not externally, but internally to the brain. In this way, developmental diaschisis of information flow to the neocortex could lead to long-term effects that resemble those of severe early-life deprivation, even under normal environmental conditions.
Contributions of the cerebellum to learning and plasticity
In the control of movement, the cerebellum has been suggested to provide an internal model needed to provide outputs that refine the accuracy of a movement. In the cognitive domain, the cerebellum might refine the accuracy of mental operations (Ito, 2008). The uniform microarchitecture of cerebellar circuitry suggests the possibility that both domains are governed by a common computational model.
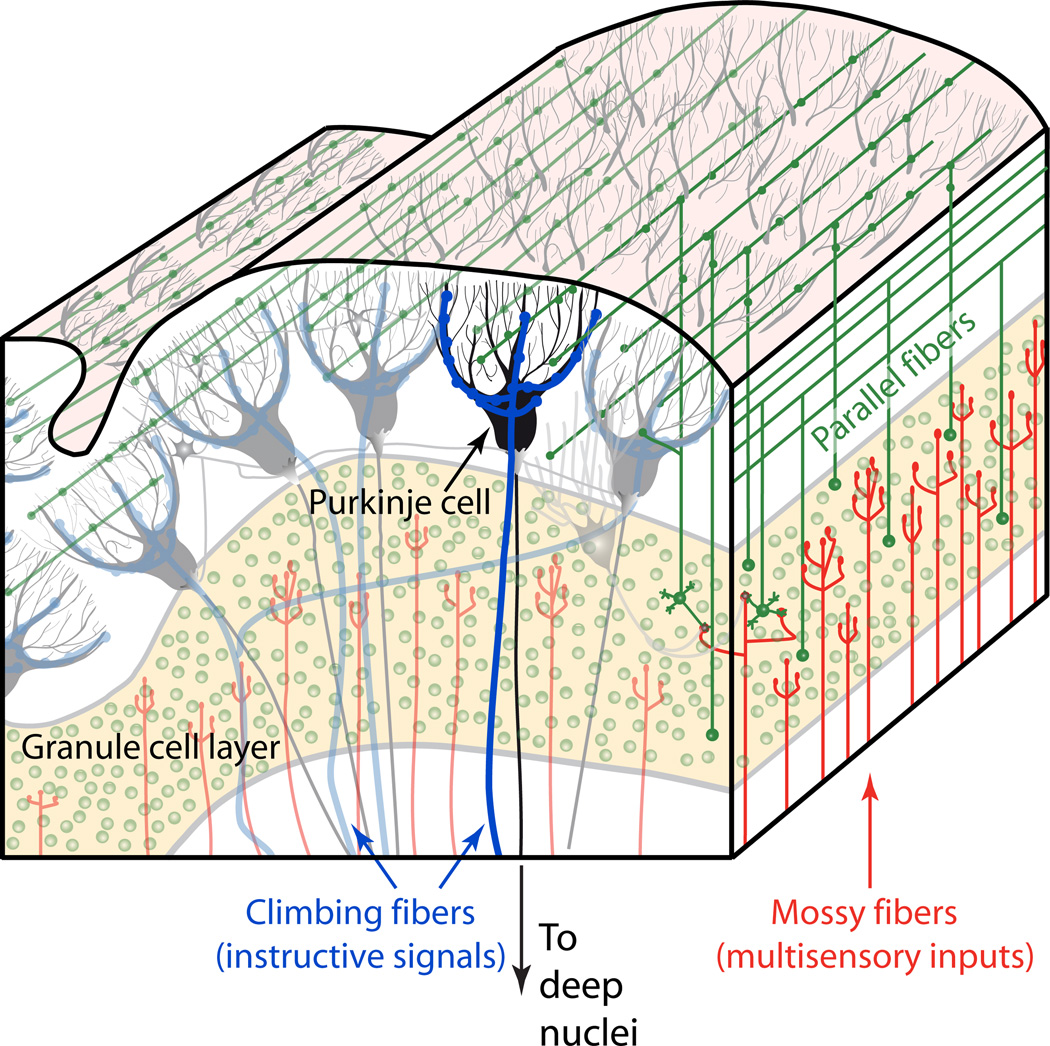
The cerebellum is widely believed to be a site for supervised learning (Figure 5). Unexpected events are thought to be signaled via the inferior olive’s climbing fibers, which strongly innervate Purkinje cells to drive a dendritic calcium-based action potential. This dendrite-specificity allows the instructive signal to be separated from the effects of the mossy fiber pathway, which drives the Purkinje cells’ output, somatic and axonal sodium spikes. The climbing fiber signal drives plasticity of a high-dimensional input from the mossy fiber pathway, the feedforward architecture of which is well suited to support the supervised learning of specific mossy fiber/granule cell patterns (Raymond et al., 1996) carrying predictive value on a subsecond time scale. The learned information is then transferred to the deep nuclei. Such architecture is capable of fine discrimination of stimulus features and transforming multisensory information to predictive output.
Figure 5. Circuitry for instructed learning in the cerebellar cortex.
Purkinje cells (black) receive the two major excitatory streams of input to the cerebellum: the mossy fibers (red), which synapse onto granule cells (green); and climbing fibers (blue). Mossy fibers and climbing fibers also send collaterals to the cerebellar deep nuclei. Cerebellar granule cells represent approximately half the neurons of the rodent or primate brain, and convey sensory, motor efference, and other information to1 the cerebellar cortex. They give rise to parallel fibers (green) which then converge massively onto Purkinje cells. Climbing fibers act as an instructive signal that can drive plasticity at recently active parallel fiber synapses. In this way, learning in the cerebellar cortex can integrate multiple sensory modalities with precise timing in the subsecond range. The sole output of the cerebellar cortex is Purkinje cell inhibition to the cerebellar deep nuclei, which in turn project to thalamus and many other brain regions.
Multisensory learning tasks requiring the cerebellum are well studied in the motor domain. Delay eyeblink conditioning (Raymond et al., 1996) is a form of learning in which an initially neutral stimulus (e.g. a tone or a light flash) becomes associated with a strong teaching stimulus (corneal airpuff or periorbital shock) that, by itself, evokes an unconditioned blink response. The unconditioned stimulus is conveyed to cerebellar cortex and nuclei via the inferior olive and its climbing fibers. Neutral (conditioned) stimuli are conveyed to the cerebellum via the mossy fiber pathway. After hundreds of closely timed pairings of conditioned and unconditioned stimuli, eventually the conditioned stimulus alone evokes a blink at the predicted time of the unconditioned stimulus. In this paradigm, different modalities (e.g. corneal airpuff and tone) are associated with one another. The cerebellum plays a similar role in vestibulo-ocular reflex gain adaptation (vestibular input and retinal slip signal).
In the case of social learning, making sensory discriminations and predictions is important because most sensory information initially has little intrinsic social valence (Cohn and Tronick, 1987). A mother’s smile is unlikely to be intrinsically rewarding to a baby, but instead must be paired with other information such as food or touch (Stack and Muir, 1992). Similarly, play also delivers social rewards whose value must be learned (Panksepp et al., 1985). It has been postulated that in autism, difficulties attending to socially salient stimuli may arise from an impairment of assigning reward to the stimuli (Dawson et al., 2002). Broadly, the cerebellum’s potential role in acquiring the ability to associate a sensory pattern with an innately rewarding or aversive event, can contribute to information processing – and even drive learning – in the neocortex. Such a role is potentially supported by the monosynaptic projections that lead from the cerebellar nuclei to VTA.
For associating an innately rewarding stimulus with other sensory events, a principal computational function of the cerebellum might be that of detecting closely-timed associations. Eyeblink conditioning and vestibulo-ocular reflex gain control have specific timing requirements for the instructive (climbing fiber) stimulus to come within a few tenths of a second after the learned (mossy fiber) stimulus. This timing relationship is reflected in the temporal order requirements for parallel fiber-Purkinje cell synaptic plasticity (Wang et al., 2000). Thus a major computational function of the cerebellum in social learning might be the ability to associate a fast social cue (sensory feedback) with a reward-outcome. In this way, the role of cerebellum in processing timing information across sensory modalities (D'Angelo and De Zeeuw, 2009) might be of specific relevance for social learning.
Cerebellar supervised learning has also been proposed as a means of acquiring internal models (both forward and inverse) about the environment (Wolpert et al., 1998). The cerebral cortex can also learn predictive models of the environment, but it does so using very different circuit architecture, one that is rich in loops and recurrent excitation. Due to these markedly contrasting architectures, it has been proposed that the cerebellar and cerebral cortex differ in their learning algorithm, with the cerebellum performing supervised learning, while the cerebral cortex performs unsupervised learning (Doya, 1999). The cerebellum thus may play a complementary functional role to neocortex, whether in motor or nonmotor function (Ito, 2008). If the cerebellum and neocortex are suited for different types of learning, cerebello-thalamo-cortical loops would provide a substrate for tasks to be processed by two very different architectures working together. Such hybrid architecture is potentially quite powerful, as it could combine the strengths of the two respective learning approaches.
Indeed, patterns learned by one structure could be passed to the other structure. Such transfer is an example of memory consolidation. In memory consolidation, the acquisition of a memory requires rapid adaptation in one brain region coupled with gradual plasticity in a second brain region, where the memory is stored (Krakauer and Shadmehr, 2006). This process has been observed between the cerebellar cortex and the deep cerebellar nuclei for cerebellum-dependent behaviors such as eyeblink conditioning, in which the expression of motor memory (but not its timing) comes to require the interposed nucleus but not the cerebellar cortex (Attwell et al., 2002). A similar process has been observed for motor skill learning, in which the motor cortex consolidates input from the cerebellum after many trials of learning (Krakauer and Shadmehr, 2006). Thus changes in cerebellum may, over time, drive changes in corresponding cortical areas.
For example, regions encompassing lobules VI and VII, where abnormalities have been reported in ASD (Carper and Courchesne, 2000), show strong covariation of resting-state functional connectivity with contralateral mid-frontal regions that appear to encompass ACC (Buckner et al., 2011). ACC roles include motivating and attending responses, detecting errors to those responses, and switching flexibly between cognitive and affective tasks (Devinsky et al., 1995). Autistic persons show deficits in response monitoring, making adjustments to optimize outcome, and the ability to monitor one’s self (Mundy, 2003), and ASD patients who score high on repetitive behavior show abnormal signaling in rostral ACC (Thakkar et al., 2008). We suggest that during development, ACC and lobule VI-VII may pass information to one another as part of the acquisition of emotional and social capacities.
The timing of cerebellar maturation is also consistent with the developmental diaschisis hypothesis. The cerebellum reaches its mature volume within months of birth in humans (Rice and Barone, 2000). In humans the cerebellum develops throughout pregnancy with rapid growth in the third trimester and in the first postnatal year (Limperopoulos et al., 2007; ten Donkelaar et al., 2003; Zervas et al., 2005). Cerebellar circuitry is vulnerable in the days and weeks following birth (ten Donkelaar et al., 2003), a period during which the cellular makeup and the quantity of inputs changes quickly (Wang and Zoghbi, 2001) and ASD genes are co-expressed in cerebellum (Willsey et al., 2013). In contrast, cortical areas continue to mature for a longer period of years (Rice and Barone, 2000). Thus, the cerebellum grows during a period of known genetic and environmental vulnerability, and reaches full size in time to potentially guide the refinement of neocortical structures.
A sensory hypothesis of cerebellar contributions to developmental disorders
Just as sensory areas are organized by experience, cognitive and social processing may also be guided by structures that process sensory and other internally-generated information to extract useful parameters. In this context, the cerebellum, which is thought to integrate sensory information (Bower, 1997) to modulate movement (Thach et al., 1992), is a candidate to play a similar role in nonmotor function (Ito, 2008). The architecture of the cerebellum appears to be well suited to learn to make fine discriminations, especially in the domain of multisensory learning. Such learning might be of considerable use to the social and cognitive brain, as a coprocessor to other brain structures (D'Angelo and Casali, 2012).
Language acquisition and the formation of social capacities are among the hardest problems that the human brain must solve, yet most babies master them effortlessly (Meltzoff et al., 2009). For example, extracting the structure contained in language requires statistical learning and attending to a wide variety of nonverbal cues (Romberg and Saffran, 2010). In both language and social development, considerable meaning is carried in the juxtaposition of events from multiple senses occurring on short time scales. In this process, supervised learning of temporal relationships by the cerebellum may play an essential role.
The cerebellum integrates many converging multimodal sensory inputs via the mossy fiber pathway, which converge with unexpected events as transmitted by the climbing fiber pathway. Mossy fibers synapse onto cerebellar granule cells, which comprise approximately half the neurons of the human brain. Many different sensory receptive fields are found near one another in the granule cell layer; as granule cell axons give rise to parallel fibers, multisensory information is thoroughly mixed and distributed across many Purkinje cells. Notably, ASD-gene-associated co-expression networks have the strongest expression in the cerebellar granule layer (Menashe et al., 2013), where information from multiple sensory modalities can be integrated (Huang et al., 2013). Thus, multisensory integrative tasks would be one area where ASD and cerebellar function may meet.
It should be emphasized at this point that the foregoing framework does not require triggers of developmental diaschisis to be exclusively cerebellar in origin. Similar consequeces would be expected for any early-life brain defect that impeded statistical learning mechanisms. Abnormal processing of any type that affected necessary sensory integration could impede early-life cognitive development. In this way a variety of subcortical abnormalities could lead to ASD.
Multisensory defects in ASD suggest cerebellar dysfunction
Atypical sensory responsiveness in ASD children can be detected as early as 4–6 months of age (Zwaigenbaum et al., 2005). Autistic individuals show abnormalities in eyeblink conditioning (Oristaglio et al., 2013; Sears et al., 1994; Tobia and Woodruff-Pak, 2009). Mouse models of ASD also show disrupted eyeblink conditioning, including Fragile X (Koekkoek et al., 2005). Thus multisensory learning deficits appear to be recurring features of both human ASD and animal models of ASD.
Consistent with the importance of subcortical sensory processing is the observation that from infancy onward, autistic children (and often their siblings) show atypical sensory responsiveness (Markram and Markram, 2010). Visual orienting latencies to nonsocial stimuli are atypically slow in 7-month-olds who later meet ASD criteria (Elison et al., 2013). At later ages, sensory abnormalities persist (Leekam et al., 2007) and more complex deficits emerge. Klin and colleagues (Klin et al., 2009) reported that 2-year-old autistic children attended more strongly to multisensory simple synchrony than more complex combinations associated with natural biological motion. ASD patients show unreliable evoked neocortical responses to simple, nonsocial sensory stimuli (Dinstein et al., 2012). These abnormalities are potentially causative, since sensory responsiveness and social symptoms are strongly correlated in high-functioning autism patients (Hilton et al., 2010). Consistent with a cerebellar-dysfunction hypothesis, motor dysfunctions are large (effect size = 1.2) and occur at a rate of approximately 80% in ASD (Fournier et al., 2010). Together, this evidence suggests that abnormal sensory preprocessing may arise early in the etiology of ASD, and perhaps play a causative role.
Finally, cerebellar learning deficits could affect not only sensory information arriving in cerebellar cortex, but could also disrupt the processing of nonsensory information. The cerebellar mossy fiber pathway has a considerable corticopontine component, which conveys efference copy for motor – and perhaps nonmotor (Huang et al., 2013) – information. Considering the self-similarity of cerebellar circuitry, any cerebellar deficits would be expected to have similar effects on the processing of all information arriving via the mossy fiber pathway.
A roadmap for testing the developmental diaschisis hypothesis
Although it seems likely that the cerebellum shapes cognitive and affective domains during development, the evidence to date comes largely from lesion experiments and clinical observations. These results provide a starting point and an opportunity to use newer and more powerful tools to map, image, and manipulate brain circuitry. The development of these tools is likely to accelerate with projects such as the BRAIN (Brain Research through Advancing Innovative Neurotechnologies) Initiative in the United States. Technologies drawn from molecular biology, physical sciences and engineering, statistics, and computation (Bargmann, 2013; Sun et al., 2012) will enable probing nonmotor and developmental roles of the cerebellum to new scientific depths.
Tests of the developmental diaschisis hypothesis fall into the following three categories. These tests can be conducted to probe a variety of upstream triggers: not only the cerebellum, but also other brain structures, as well as specific molecular defects associated with genetic susceptibility loci.
(1) Does early-life disruption of upstream triggers have selective effects on adult cognitive and affective function?
Lesion experiments are irreversible and are defined spatially, encompassing all cells within reach of a burn or chemical injection. It is now possible to express inactivating receptors (Asrican et al., 2013; Nielsen et al., 2012; Wess et al., 2013) in specific cell populations, which do not act unless exposed to a ligand or light. These tools can target defined nuclei and cell types and are reversible on time scales into the subsecond range. Inactivation can even be performed during specific behaviors. Such tools can be used to test when in development, and under what circumstances, a region perturbs cognitive and affective behavior and disrupts the anatomy and physiological function of neural circuitry at remote sites. In the case of cerebellum, it should be possible to target specific subregions (for instance lobules VI/VII) and cell types, as well as smaller structures throughout the brain (for instance the deep nuclear-VTA pathway).
(2) In adult life, do specific cerebellar regions have specific influences on remote counterparts in neocortex, and vice versa?
One major technological priority for the BRAIN initiative is monitoring neural activity at multiple regions. Already, neural activity can be optically monitored in behaving mice using two-photon microscopy and whole-cell single cell recording. Social and cognitive interactions can be probed using head-fixed tasks or using miniaturized microscopes in freely-moving animals.
Once specific brain locations and cell types of interest have been identified, it will be useful to map the exact circuitry to which the location connects throughout the brain. A variety of transsynaptic tools have been developed to achieve controlled labeling of circuitry. Reconstruction is somewhat limited by the time required for sectioning and tracing. Tissue-clearing and automated tracing methods should allow viral tracing efforts to be accelerated considerably. As an example, it would be of great interest to know what type of information is passed between lobules VI/VII and ACC. ACC is thought to participate in exploration and exploitation of an animal's environment, and the role of cerebellar learning and discriminative sensory processing in this process remains to be investigated. It will also be of interest to compare normal and pathological interactions in both human disease and in mouse models, not just for autism but also other disorders with a cognitive component.
(3) Is the upstream region a potential target for rescue in mouse models of developmental disorders?
The availability of mouse models for developmental disorders opens the possibility that adult dysfunctions in these animals could be rescued by early-life interventions. If dysfunction in an identified circuit drives the maturation of brain circuitry off track, it might be possible to rescue a normal trajectory by boosting or restoring the circuit’s function. Tests of rescue should include behavioral, anatomical, and circuit functional measures. Optogenetic, pharmacogenetic, flexible electrode, or noninvasive technologies could be used to inactivate or enhance the output or effectiveness of an upstream brain region such as the cerebellar cortex or nuclei.
In addition, until recently it was believed that once a sensitive period closes further modifications to the circuit become extremely difficult if not impossible. Recently, however, it was shown that critical periods can be reopened (Bavelier et al., 2010). It remains to be determined whether this is true for social capacities, which would be expected to have later sensitive periods.
In summary, we propose that the concept of developmental diaschisis may be of general utility in the understanding of pediatric neurology, in which it is commonplace knowledge that early-life damage to a brain region can have very different consequences than adult cases (Stiles et al., 2005; Swaiman, 2012). Bridging this gap requires new experimental tests to fill in a conceptual framework for how the cerebellum may guide other regions in the process of constructing the functions of the brain.
ACKNOWLEDGMENTS
We thank Benjamin Campbell, Sara Connolly, Cristina Domnisoru, Jeff Erlich, Daniel Geschwind, Elizabeth Gould, David Heeger, Mala Murthy, Bence Ölveczky, Jordan Taylor, Huda Zoghbi, and members of the Wang lab for discussion and comments. We thank Randy Buckner and Partha Mitra for the use of images. This work was supported by NIH R01 NS045193 (S.W.), NIH F31 MH098651 (A.K.), the Nancy Lurie Marks Family Foundation (S.W.), and the Sutherland Cook Fund (S.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abell F, Krams M, Ashburner J, Passingham R, Friston K, Frackowiak R, Happe F, Frith C, Frith U. The neuroanatomy of autism: a voxel-based whole brain analysis of structural scans. Neuroreport. 1999;10:1647–1651. doi: 10.1097/00001756-199906030-00005. [DOI] [PubMed] [Google Scholar]
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Afif S, Staden M, Krauss JK, Schwabe K, Hermann EJ. Splitting of the cerebellar vermis in juvenile rats--effects on social behavior, vocalization and motor activity. Behav Brain Res. 2013;250:293–298. doi: 10.1016/j.bbr.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Allin M, Matsumoto H, Santhouse AM, Nosarti C, AlAsady MH, Stewart AL, Rifkin L, Murray RM. Cognitive and motor function and the size of the cerebellum in adolescents born very pre-term. Brain. 2001;124:60–66. doi: 10.1093/brain/124.1.60. [DOI] [PubMed] [Google Scholar]
- Apps R, Hawkes R. Cerebellar cortical organization: a one-map hypothesis. Nat Rev Neurosci. 2009;10:670–681. doi: 10.1038/nrn2698. [DOI] [PubMed] [Google Scholar]
- Asrican B, Augustine GJ, Berglund K, Chen S, Chow N, Deisseroth K, Feng G, Gloss B, Hira R, Hoffmann C, Kasai H, Katarya M, Kim J, Kudolo J, Lee LM, Lo SQ, Mancuso J, Matsuzaki M, Nakajima R, Qiu L, Tan G, Tang Y, Ting JT, Tsuda S, Wen L, Zhang X, Zhao S. Next-generation transgenic mice for optogenetic analysis of neural circuits. Front Neural Circuits. 2013;7:160. doi: 10.3389/fncir.2013.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40:1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- Attwell PJ, Ivarsson M, Millar L, Yeo CH. Cerebellar mechanisms in eyeblink conditioning. Ann N Y Acad Sci. 2002;978:79–92. doi: 10.1111/j.1749-6632.2002.tb07557.x. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Loveland KA. The orbitofrontal-amygdala circuit and selfregulation of social-emotional behavior in autism. Neurosci Biobehav Rev. 2006;30:97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Machado CJ, Kazama A. Behavioral outcomes of late-onset or early-onset orbital frontal cortex (areas 11/13) lesions in rhesus monkeys. Ann N Y Acad Sci. 2011;1239:71–86. doi: 10.1111/j.1749-6632.2011.06211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P. A clinicopathological study of autism. Brain. 1998;121(Pt 5):889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Bargmann C, Newsome W, Anderson D, Brown E, Deisseroth K, Donoghue J, MacLeish P, Marder E, Normann R, Sanes J, Schnitzer M, Sejnowski T, Tank D, Tsien R, Ugurbil K. Advisory Committee to the NIH Director Interim Report: Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Intiative Working Group. 2013 [Google Scholar]
- Baudouin SJ, Gaudias J, Gerharz S, Hatstatt L, Zhou K, Punnakkal P, Tanaka KF, Spooren W, Hen R, De Zeeuw CI, Vogt K, Scheiffele P. Shared synaptic pathophysiology in syndromic and nonsyndromic rodent models of autism. Science. 2012;338:128–132. doi: 10.1126/science.1224159. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Bolton P, Wheelwright S, Scahill V, Short L, Mead G, Smith A. Does autism occur more often in families of physicists, engineers, and mathematicians? Autism. 1998;2:296–301. [Google Scholar]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC. The amygdala theory of autism. Neurosci Biobehav Rev. 2000;24:355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Basson MA, Wingate RJ. Congenital hypoplasia of the cerebellum: developmental causes and behavioral consequences. Front Neuroanat. 2013;7:29. doi: 10.3389/fnana.2013.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci. 2010;30:14964–14971. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker EBE, Stoodley CJ. Autism spectrum disorder and the cerebellum. Int Rev Neurobiol. 2013;113:1–34. doi: 10.1016/B978-0-12-418700-9.00001-0. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar S, Chahrour M, Thaller C, Shaw CA, Zoghbi HY. Mouse models of MeCP2 disorders share gene expression changes in the cerebellum and hypothalamus. Hum Mol Genet. 2009;18:2431–2442. doi: 10.1093/hmg/ddp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beversdorf DQ, Manning SE, Hillier A, Anderson SL, Nordgren RE, Walters SE, Nagaraja HN, Cooley WC, Gaelic SE, Bauman ML. Timing of prenatal stressors and autism. J Autism Dev Disord. 2005;35:471–478. doi: 10.1007/s10803-005-5037-8. [DOI] [PubMed] [Google Scholar]
- Bliss-Moreau E, Bauman MD, Amaral DG. Neonatal amygdala lesions result in globally blunted affect in adult rhesus macaques. Behav Neurosci. 2011;125:848–858. doi: 10.1037/a0025757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobée S, Mariette E, Tremblay-Leveau H, Caston J. Effects of early midline cerebellar lesion on cognitive and emotional functions in the rat. Behav Brain Res. 2000;112:107–117. doi: 10.1016/s0166-4328(00)00166-2. [DOI] [PubMed] [Google Scholar]
- Bolduc ME, du Plessis AJ, Sullivan N, Guizard N, Zhang X, Robertson RL, Limperopoulos C. Regional cerebellar volumes predict functional outcome in children with cerebellar malformations. Cerebellum. 2012;11:531–542. doi: 10.1007/s12311-011-0312-z. [DOI] [PubMed] [Google Scholar]
- Boltshauser E. Cerebellum-small brain but large confusion: a review of selected cerebellar malformations and disruptions. Am J Med Genet A. 2004;126A:376–385. doi: 10.1002/ajmg.a.20662. [DOI] [PubMed] [Google Scholar]
- Bostan AC, Strick PL. The cerebellum and basal ganglia are interconnected. Neuropsychol Rev. 2010;20:261–270. doi: 10.1007/s11065-010-9143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JM. Control of sensory data acquisition. Int Rev Neurobiol. 1997;41:489–513. doi: 10.1016/s0074-7742(08)60367-0. [DOI] [PubMed] [Google Scholar]
- Brielmaier J, Matteson PG, Silverman JL, Senerth JM, Kelly S, Genestine M, Millonig JH, DiCicco-Bloom E, Crawley JN. Autism-relevant social abnormalities and cognitive deficits in engrailed-2 knockout mice. PLoS ONE. 2012;7:e40914. doi: 10.1371/journal.pone.0040914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BC, Wang SS-H. Familial linkage between neuropsychiatric disorders and intellectual interests. PLoS ONE. 2012;7:e30405. doi: 10.1371/journal.pone.0030405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carper RA, Courchesne E. Inverse correlation between frontal lobe and cerebellum sizes in children with autism. Brain. 2000;123(Pt 4):836–844. doi: 10.1093/brain/123.4.836. [DOI] [PubMed] [Google Scholar]
- Chlebowski C, Robins DL, Barton ML, Fein D. Large-scale use of the modified checklist for autism in low-risk toddlers. Pediatrics. 2013;131:e1121–e1127. doi: 10.1542/peds.2012-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow TW. Personality in frontal lobe disorders. Curr Psychiatry Rep. 2000;2:446–1451. doi: 10.1007/s11920-000-0031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DA, Mitra PP, Wang SS-H. Scalable architecture in mammalian brains. Nature. 2001;411:189–193. doi: 10.1038/35075564. [DOI] [PubMed] [Google Scholar]
- Cohn JF, Tronick EZ. Mother infant face-to-face interaction - the sequence of dyadic states at 3,6, and 9 months. Dev Psychol. 1987;23:68–77. [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schriebman L, Haas RH, Akshoomoff NA, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, Barnes CC, Pierce K. Neuron number and size in prefrontal cortex of children with autism. JAMA. 2011;306:2001–2010. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Redcay E, Morgan JT, Kennedy DP. Autism at the beginning: microstructural and growth abnormalities underlying the cognitive and behavioral phenotype of autism. Dev Psychopathol. 2005;17:577–597. doi: 10.1017/S0954579405050285. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Webb SJ, Schumann CM. From toddlers to adults: the changing landscape of the brain in autism. In: Amaral D, Geschwind D, Dawson G, editors. Autism Spectrum Disorders. Oxford: Oxford University Press; 2011. pp. 611–631. [Google Scholar]
- Courchesne E, Yeung-Courchesne R, Press GA, Hesselink JR, Jernigan TL. Hypoplasia of cerebellar vermal lobules VI and VII in autism. The N Engl J Med. 1988;318:1349–1354. doi: 10.1056/NEJM198805263182102. [DOI] [PubMed] [Google Scholar]
- D'Angelo E, Casali S. Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition. Front Neural Circuits. 2012;6:116. doi: 10.3389/fncir.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo E, De Zeeuw CI. Timing and plasticity in the cerebellum: focus on the granular layer. Trends Neurosci. 2009;32:30–40. doi: 10.1016/j.tins.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Daniels AM, Halladay AK, Shih A, Elder LM, Dawson G. Approaches to enhancing the early detection of autism spectrum disorders: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2014;53:141–152. doi: 10.1016/j.jaac.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Dawson G, Carver L, Meltzoff AN, Panagiotides H, McPartland J, Webb SJ. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Dev. 2002;73:700–717. doi: 10.1111/1467-8624.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Casey BJ, Dahl RE, Birmaher B, Williamson DE, Thomas KM, Axelson DA, Frustaci K, Boring AM, Hall J, Ryan ND. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2000;48:51–57. doi: 10.1016/s0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Pegado F, Braga LW, Ventura P, Nunes Filho G, Jobert A, Dehaene-Lambertz G, Kolinsky R, Morais J, Cohen L. How learning to read changes the cortical networks for vision and language. Science. 2010;330:1359–1364. doi: 10.1126/science.1194140. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dinstein I, Heeger DJ, Lorenzi L, Minshew NJ, Malach R, Behrmann M. Unreliable evoked responses in autism. Neuron. 2012;75:981–991. doi: 10.1016/j.neuron.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doya K. What are the computations of the cerebellum, the basal ganglia and the cerebral cortex? Neural Netw. 1999;12:961–974. doi: 10.1016/s0893-6080(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Elison JT, Paterson SJ, Wolff JJ, Reznick JS, Sasson NJ, Gu H, Botteron KN, Dager SR, Estes AM, Evans AC, Gerig G, Hazlett HC, Schultz RT, Styner M, Zwaigenbaum L, Piven J IBIS Network. White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. Am J Psychiatry. 2013;170:899–908. doi: 10.1176/appi.ajp.2012.12091150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegood J, Pacey LK, Hampson DR, Lerch JP, Henkelman RM. Anatomical phenotyping in a mouse model of fragile X syndrome with magnetic resonance imaging. Neuroimage. 2010;53:1023–1029. doi: 10.1016/j.neuroimage.2010.03.038. [DOI] [PubMed] [Google Scholar]
- Ellegood J, Markx S, Lerch JP, Steadman PE, Genç C, Provenzano F, Kushner SA, Henkelman RM, Karayiorgou, Gogos JA. Neuroanatomical phenotypes in a mouse model of the 22q11.2 microdeletion. Mol Psychiatry. 2014;19:99–107. doi: 10.1038/mp.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslinger PJ, Flaherty-Craig CV, Benton AL. Developmental outcomes after early prefrontal cortex damage. Brain and Cognition. 2004;55:84–103. doi: 10.1016/S0278-2626(03)00281-1. [DOI] [PubMed] [Google Scholar]
- Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH. Motor coordination in autism spectrum disorders: a synthesis and meta-analysis. J Autism Dev Disord. 2010;40:1227–1240. doi: 10.1007/s10803-010-0981-3. [DOI] [PubMed] [Google Scholar]
- Friel KM, Martin JH. Bilateral activity-dependent interactions in the developing corticospinal system. J Neurosci. 2007;27:11083–11090. doi: 10.1523/JNEUROSCI.2814-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgliani JE. Master’s thesis, University of Pittsburgh. 2012. Vermis-cingulate cortex interconnections: a cerebro-cerebellar circuit in the rat. [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J Comp Neurol. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Girgis RR, Minshew NJ, Melhern NM, Nutche JJ, Keshavan MS, Hardan AY. Volumetric alterations of the orbitofrontal cortex in autism. Prog Neuropsychopharmacol. 2007;31:41–45. doi: 10.1016/j.pnpbp.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasson EJ, Bower C, Petterson B, de Klerk N, Chaney G, Hallmayer JF. Perinatal factors and the development of autism: a population study. Arch Gen Psychiatry. 2004;61:618–627. doi: 10.1001/archpsyc.61.6.618. [DOI] [PubMed] [Google Scholar]
- Gudsnuk KM, Champagne FA. Epigenetic effects of early developmental experiences. Clin Perinatol. 2011;38:703–717. doi: 10.1016/j.clp.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Hadland KA, Rushworth MF, Gaffan D, Passingham RE. The effect of cingulate lesions on social behaviour and emotion. Neuropsychologia. 2003;41:919–931. doi: 10.1016/s0028-3932(02)00325-1. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Tayama M, Murakawa K, Yoshimoto T, Miyazaki M, Harada M, Kuroda Y. Development of the brainstem and cerebellum in autistic patients. J Autism Dev Disord. 1995;25:1–18. doi: 10.1007/BF02178163. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hilton CL, Harper JD, Kueker RH, Lang AR, Abbacchi AM, Todorov A, LaVesser PD. Sensory responsiveness as a predictor of social severity in children with high functioning autism spectrum disorders. J Autism Dev Disord. 2010;40:937–945. doi: 10.1007/s10803-010-0944-8. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- Huang CC, Sugino K, Shima Y, Guo C, Bai S, Mensh BD, Nelson SB, Hantman AW. Convergence of pontine and proprioceptive streams onto multimodal cerebellar granule cells. eLife. 2013;2:e00400. doi: 10.7554/eLife.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9:304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic Disturbances of Affective Contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Kaufmann WE, Cooper KL, Mostofsky SH, Capone GT, Kates WR, Newschaffer CJ, Bukelis I, Stump MH, Jann AE, Lanham DC. Specificity of cerebellar vermian abnormalities in autism: a quantitative magnetic resonance imaging study. J Child Neurol. 2003;18:463–470. doi: 10.1177/08830738030180070501. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney DK, Miller AM, Crowley DJ, Huang E, Gerber E. Autism prevalence following prenatal exposure to hurricanes and tropical storms in Louisiana. J Autism Dev Disord. 2008a;38:481–488. doi: 10.1007/s10803-007-0414-0. [DOI] [PubMed] [Google Scholar]
- Kinney DK, Munir KM, Crowley DJ, Miller AM. Prenatal stress and risk for autism. Neurosci Biobehav Rev. 2008b;32:1519–1532. doi: 10.1016/j.neubiorev.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459:257–261. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Koekkoek SK, Yamaguchi K, Milojkovic BA, Dortland BR, Ruigrok TJ, Maex R, De Graaf W, Smit AE, VanderWerf F, Bakker CE, Willemsen R, Ikeda T, Kakizawa S, Onodera K, Nelson DL, Mientjes E, Joosten M, De Schutter E, Oostra BA, Ito M, De Zeeuw CI. Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in Fragile X syndrome. Neuron. 2005;47:339–352. doi: 10.1016/j.neuron.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Koziol LF, Budding D, Andreasen N, D'Arrigo S, Bulgheroni S, Imamizu H, Ito M, Manto M, Marvel C, Parker K, Pezzulo G, Ramnani N, Riva D, Schmahmann J, Vandervert L, Yamazaki T. Consensus paper: the cerebellum's role in movement and cognition. Cerebellum. 2014;13:151–177. doi: 10.1007/s12311-013-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Shadmehr R. Consolidation of motor memory. Trends Neurosci. 2006;29:58–64. doi: 10.1016/j.tins.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19:2485–2497. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond CS, Heinrich J, Delorme R, Proepper C, Betancur C, Huguet G, Konyukh M, Chaste P, Ey E, Rastam M, Anckarsäter H, Nygren G, Gillberg IC, Melke J, Toro R, Regnault B, Fauchereau F, Mercati O, Lemière N, Skuse D, Poot M, Holt R, Monaco AP, Järvelä I, Kantojärvi K, Vanhala R, Curran S, Collier DA, Bolton P, Chiocchetti A, Klauck SM, Poustka F, Freitag CM, Waltes R, Kopp M, Duketis E, Bacchelli E, Minopoli F, Ruta L, Battaglia A, Mazzone L, Maestrini E, Sequeira AF, Oliveira B, Vicente A, Oliveira G, Pinto D, Scherer SW, Zelenika D, Delepine M, Lathrop M, Bonneau D, Guinchat V, Devillard F, Assouline B, Mouren MC, Leboyer M, Gillberg C, Boeckers TM, Bourgeron T. Genetic and functional analyses of SHANK2 mutations suggest a multiple hit model of autism spectrum disorders. PLoS Genet. 2012;8:e1002521. doi: 10.1371/journal.pgen.1002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leekam SR, Nieto C, Libby SJ, Wing L, Gould J. Describing the sensory abnormalities of children and adults with autism. J Autism Dev Disord. 2007;37:894–910. doi: 10.1007/s10803-006-0218-7. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C, Bassan H, Gauvreau K, Robertson RL, Jr, Sullivan NR, Benson CB, Avery L, Stewart J, Soul JS, Ringer SA, Volpe JJ, du Plessis AJ. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. 2007;120:584–593. doi: 10.1542/peds.2007-1041. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C, Chilingaryan G, Sullivan N, Guizard N, Robertson RL, du Plessis AJ. Injury to the premature cerebellum: outcome is related to remote cortical development. Cereb Cortex. 2014;24:728–736. doi: 10.1093/cercor/bhs354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- Magnusson C, Rai D, Goodman A, Lundberg M, Idring S, Svensson A, Koupil I, Serlachius E, Dalman C. Migration and autism spectrum disorder: population-based study. Br J Psychiatry. 2012;201:109–115. doi: 10.1192/bjp.bp.111.095125. [DOI] [PubMed] [Google Scholar]
- Málková L, Mishkin M, Suomi SJ, Bachevalier J. Long-term effects of neonatal medial temporal ablations on socioemotional behavior in monkeys (Macaca mulatta) Behav Neurosci. 2010;124:742–760. doi: 10.1037/a0021622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariën P, Ackermann H, Adamaszek M, Barwood CH, Beaton A, Desmond J, De Witte E, Fawcett AJ, Hertrich I, Kuper M, Leggio M, Marvel C, Molinari M, Murdoch BE, Nicolson RI, Schmahmann JD, Stoodley CJ, Thürling M, Timmann D, Wouters E, Ziegler W. Consensus paper: Language and the cerebellum: an ongoing enigma. Cerebellum. 2014;13:386–410. doi: 10.1007/s12311-013-0540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Markram K. The intense world theory – a unifying theory of the neurobiology of autism. Front Hum Neurosci. 2010;4:224. doi: 10.3389/fnhum.2010.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN, Kuhl PK, Movellan J, Sejnowski TJ. Foundations for a new science of learning. Science. 2009;325:284–288. doi: 10.1126/science.1175626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menashe I, Grange P, Larsen EC, Banerjee-Basu S, Mitra PP. Co-expression profiling of autism genes in the mouse brain. PLoS Comput Biol. 2013;9:e1003128. doi: 10.1371/journal.pcbi.1003128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Hariri AR, Munoz KE, Mervis CB, Mattay VS, Morris CA, Berman KF. Neural correlates of genetically abnormal social cognition in Williams syndrome. Nat Neurosci. 2005;8:991–993. doi: 10.1038/nn1494. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Sullivan RM. Rodent model of infant attachment learning and stress. Dev Psychobiol. 2010;52:651–660. doi: 10.1002/dev.20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med. 2008;359:262–273. doi: 10.1056/NEJMoa0706475. [DOI] [PubMed] [Google Scholar]
- Mundy P. Annotation: the neural basis of social impairments in autism: the role of the dorsal medial-frontal cortex and anterior cingulate system. J Child Psychol Psychiatry. 2003;44:793–809. doi: 10.1111/1469-7610.00165. [DOI] [PubMed] [Google Scholar]
- Nielsen KJ, Callaway EM, Krauzlis RJ. Viral vector-based reversible neuronal inactivation and behavioral manipulation in the macaque monkey. Front Syst Neurosci. 2012;6:48. doi: 10.3389/fnsys.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak MA, Suomi SJ. Abnormal behavior in nonhuman primates and models of development. In: Burbacher GP, Sackett GP, Grant KS, editors. Primate models of children's health and developmental disabilities. Boston: Academic Press; 2008. pp. 141–160. [Google Scholar]
- O'Roak BJ, Deriziotis P, Lee C, Vives L, Schwartz JJ, Girirajan S, Karakoc E, Mackenzie AP, Ng SB, Baker C, Reilly B, Akey JM, Borenstein E, Rieder MJ, Nickerson DA, Bernier R, Shendure J, Eichler EE. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nature Genet. 2011;43:585–589. doi: 10.1038/ng.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oristaglio J, Hyman West S, Ghaffari M, Lech MS, Verma BR, Harvey JA, Welsh JP, Malone RP. Children with autism spectrum disorders show abnormal conditioned response timing on delay, but not trace, eyeblink conditioning. Neuroscience. 2013;248:708–718. doi: 10.1016/j.neuroscience.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmen SJ, van Engeland H, Hof PR, Schmitz C. Neuropathological findings in autism. Brain. 2004;127:2572–2583. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Siviy S, Normansell L. The psychobiology of play: Theoretical and methodological perspectives. Neurosci Biobehav Rev. 1985;8:465–492. doi: 10.1016/0149-7634(84)90005-8. [DOI] [PubMed] [Google Scholar]
- Parikshak NN, Luo R, Zhang A, Won H, Lowe JK, Chandran V, Horvath S, Geschwind DH. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell. 2013;155:1008–1021. doi: 10.1016/j.cell.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson PH. Maternal infection and autism. Brain Behavior Immun. 2012;26:393. doi: 10.1016/j.bbi.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Paul LK, Corsello C, Tranel D, Adolphs R. Does bilateral damage to the human amygdala produce autistic symptoms? J Neurodev Disord. 2010;2:165–173. doi: 10.1007/s11689-010-9056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson OT. Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J Comp Neurol. 1979;187:117–143. doi: 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- Piaget J. Piaget's theory. In: Kessen W, editor. Handbook of Child Psychology. New York: Wiley; 1983. pp. 103–128. [Google Scholar]
- Pope CA, 3rd, Burnett RT, Turner MC, Cohen A, Krewski D, Jerrett M, Gapstur SM, Thun MJ. Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: shape of the exposure-response relationships. Environ Health Perspect. 2011;119:1616–1621. doi: 10.1289/ehp.1103639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevosto V, Graf W, Ugolini G. Cerebellar inputs to intraparietal cortex areas LIP and MIP: functional frameworks for adaptive control of eye movements, reaching, and arm/eye/head movement coordination. Cereb Cortex. 2010;20:214–228. doi: 10.1093/cercor/bhp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JL, Lisberger SG, Mauk MD. The cerebellum: a neuronal learning machine? Science. 1996;272:1126–1131. doi: 10.1126/science.272.5265.1126. [DOI] [PubMed] [Google Scholar]
- Redcay E, Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol Psychiatry. 2005;58:1–9. doi: 10.1016/j.biopsych.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva D, Giorgi C. The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumours. Brain. 2000;123(Pt 5):1051–1061. doi: 10.1093/brain/123.5.1051. [DOI] [PubMed] [Google Scholar]
- Roberts AL, Koenen KC, Lyall K, Ascherio A, Weisskopf MG. Women's posttraumatic stress symptoms and autism spectrum disorder in their children. Res Aut Spectr Disord. 2014;8:608–616. doi: 10.1016/j.rasd.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaniella R, Borgatti R. Cerebellar agenesis. In: Manto M, Schmahmann JD, Rossi F, Koibuchi N, editors. Handbook of the Cerebellum and Cerebellar Disorders. Dordrecht: Springer; 2012. pp. 1855–1872. [Google Scholar]
- Romberg AR, Saffran JR. Statistical learning and language acquisition. Wiley Interdiscip Rev Cogn Sci. 2010;1:906–914. doi: 10.1002/wcs.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Andersen-Wood L, Beckett C, Bredenkamp D, Castle J, Groothues C, Kreppner J, Keaveney L, Lord C, O'Connor TG. Quasi-autistic patterns following severe early global privation. English and Romanian Adoptees (ERA) Study Team. J Child Psychol Psychiatry. 1999;40:537–549. [PubMed] [Google Scholar]
- Sadakata T, Kakegawa W, Mizoguchi A, Washida M, Katoh-Semba R, Shutoh F, Okamoto T, Nakashima H, Kimura K, Tanaka M, Sekine Y, Itohara S, Yazaki M, Nagao S, Furuichi T. Impaired cerebellar development and function in mice lacking CAPS2, a protein involved in neurotrophin release. J Neurosci. 2007;27:2472–2482. doi: 10.1523/JNEUROSCI.2279-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadakata T, Shinoda Y, Oka M, Sekine Y, Sato Y, Saruta C, Miwa H, Tanaka M, Itohara S, Furuichi T. Reduced axonal localization of a Caps2 splice variant impairs axonal release of BDNF and causes autistic-like behavior in mice. Proc Natl Acad Sci USA. 2012;109:21104–21109. doi: 10.1073/pnas.1210055109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson NJ, Lam KS, Parlier M, Daniels JL, Piven J. Autism and the broad autism phenotype: familial patterns and intergenerational transmission. J Neurodev Disord. 2013;5:11. doi: 10.1186/1866-1955-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schneider ER, Civillico EF, Wang SS-H. Calcium-based dendritic excitability and its regulation in the deep cerebellar nuclei. J Neurophysiol. 2013;109:2282–2292. doi: 10.1152/jn.00925.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Bauman MD, Amaral DG. Abnormal structure or function of the amygdala is a common component of neurodevelopmental disorders. Neuropsychologia. 2011;49:745–759. doi: 10.1016/j.neuropsychologia.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Nordahl CW. Bridging the gap between MRI and postmortem research in autism. Brain Res. 2011;1380:175–186. doi: 10.1016/j.brainres.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JA, Schumann CM, Goodlin-Jones BL, Amaral DG. A comprehensive volumetric analysis of the cerebellum in children and adolescents with autism spectrum disorder. Autism Res. 2009;2:246–257. doi: 10.1002/aur.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears LL, Finn PR, Steinmetz JE. Abnormal classical eye-blink conditioning in autism. J Autism Dev Disord. 1994;24:737–751. doi: 10.1007/BF02172283. [DOI] [PubMed] [Google Scholar]
- Smalley SL. Autism and tuberous sclerosis. J Autism Dev Disord. 1998;28:407–414. doi: 10.1023/a:1026052421693. [DOI] [PubMed] [Google Scholar]
- Smyke AT, Zeanah CH, Fox NA, Nelson CA. A new model of foster care for young children:the Bucharest early intervention project. Child Adolesc Psychiatric Clin N Am. 2009;18:721–734. doi: 10.1016/j.chc.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, Dager SR. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Stack DM, Muir DW. Adult tactile stimulation during face-to-face interactions modulates 5-month-olds’ affect and attention. Child Dev. 1992;63:1509–1525. [PubMed] [Google Scholar]
- Stanfield AC, McIntosh AM, Spencer MD, Philip R, Gaur S, Lawrie SM. Towards a neuroanatomy of autism: a systematic review and meta-analysis of structural magnetic resonance imaging studies. Eur Psychiatry. 2008;23:289–299. doi: 10.1016/j.eurpsy.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Steadman PE, Ellegood J, Szulc KU, Turnbull DH, Joyner AL, Henkelman RM, Lerch JP. Genetic effects on cerebellar structure across mouse models of autism using a magnetic resonance imaging atlas. Autism Res. 2014;7:124–137. doi: 10.1002/aur.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL, Parikshak NN, Geschwind DH. Rare inherited variation in autism: beginning to see the forest and a few trees. Neuron. 2013;77:209–211. doi: 10.1016/j.neuron.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinlin M, Wingeier K. Cerebellum and Cogniton. Dordrecht: Springer; 2013. [Google Scholar]
- Stiles J, Reilly J, Paul B, Moses P. Cognitive development following early brain injury: evidence for neural adaptation. Trends Cogn Sci. 2005;9:136–143. doi: 10.1016/j.tics.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Stoner R, Chow ML, Boyle MP, Sunkin SM, Mouton PR, Roy S, Wynshaw-Boris A, Colamarino SA, Lein ES, Courchesne E. Patches of disorganization in the neocortex of children with autism. N Engl J Med. 2014;370:1209–1219. doi: 10.1056/NEJMoa1307491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. NeuroImage. 2012;59:1560–1570. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Sun XR, Giovannucci A, Sgro AE, Wang SS-H. SnapShot: Optical Control and Imaging of Brain Activity. Cell. 2012;149:1650.e2–1650.e2. doi: 10.1016/j.cell.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Suzuki L, Coulon P, Sabel-Goedknegt EH, Ruigrok TJ. Organization of cerebral projections to identified cerebellar zones in the posterior cerebellum of the rat. J Neurosci. 2012;32:10854–10869. doi: 10.1523/JNEUROSCI.0857-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaiman KF, Ashwal S, Ferriero DM, Schor NF. Swaiman's Pediatric Neurology: Principles and Practice. 5th ed. Philadelphia: Elsevier Saunders; 2012. [Google Scholar]
- Tavano A, Grasso R, Gagliardi C, Triulzi F, Bresolin N, Fabbro F, Borgatti R. Disorders of cognitive and affective development in cerebellar malformations. Brain. 2007;130:2646–2660. doi: 10.1093/brain/awm201. [DOI] [PubMed] [Google Scholar]
- ten Donkelaar HJ, Lammens M, Wesseling P, Thijssen HO, Renier WO. Development and developmental disorders of the human cerebellum. J Neurol. 2003;250:1025–1036. doi: 10.1007/s00415-003-0199-9. [DOI] [PubMed] [Google Scholar]
- Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci. 1992;15:403–442. doi: 10.1146/annurev.ne.15.030192.002155. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Polli FE, Joseph RM, Tuch DS, Hadjikhani N, Barton JJ, Manoach DS. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD) Brain. 2008;131:2464–2478. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmann D, Brandauer B, Hermsdorfer J, Ilg W, Konczak J, Gerwig M, Gizewski ER, Schoch B. Lesion-symptom mapping of the human cerebellum. Cerebellum. 2008;7:602–606. doi: 10.1007/s12311-008-0066-4. [DOI] [PubMed] [Google Scholar]
- Timmann D, Dimitrova A, Hein-Kropp C, Wilhelm H, Dorfler A. Cerebellar agenesis: clinical, neuropsychological and MR findings. Neurocase. 2003;9:402–413. doi: 10.1076/neur.9.5.402.16555. [DOI] [PubMed] [Google Scholar]
- Tobia MJ, Woodruff-Pak DS. Delay eyeblink classical conditioning is impaired in Fragile X syndrome. Behav Neurosci. 2009;123:665–676. doi: 10.1037/a0015662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, Steinberg J, Crawley JN, Regehr WG, Sahin M. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012;488:647–651. doi: 10.1038/nature11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nat Neurosci. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, Mill J, Cantor R, Blencowe BJ, Geschwind DH. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voogd J, Schraa-Tam CK, van der Geest JN, De Zeeuw CI. Visuomotor cerebellum in human and nonhuman primates. Cerebellum. 2012;11:392–410. doi: 10.1007/s12311-010-0204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SS-H, Denk W, Häusser M. Coincidence detection in single dendritic spines mediated by calcium release. Nat Neurosci. 2000;3:1266–1273. doi: 10.1038/81792. [DOI] [PubMed] [Google Scholar]
- Wang VY, Zoghbi HY. Genetic regulation of cerebellar development. Nat Rev Neurosci. 2001;2:484–491. doi: 10.1038/35081558. [DOI] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu LS, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Watson TC, Jones MW, Apps R. Electrophysiological mapping of novel prefrontal - cerebellar pathways. Front Integr Neuroscience. 2009;3:18. doi: 10.3389/neuro.07.018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel J, Kuchna I, Nowicki K, Imaki H, Wegiel J, Marchi E, Ma SY, Chauhan A, Chauhan V, Bobrowicz TW, de Leon M, Louis LA, Cohen IL, London E, Brown WT, Wisniewski T. The neuropathology of autism: defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuropathologica. 2010;119:755–770. doi: 10.1007/s00401-010-0655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells EM, Walsh KS, Khademian ZP, Keating RF, Packer RJ. The cerebellar mutism syndrome and its relation to cerebellar cognitive function and the cerebellar cognitive affective disorder. Dev Disabil Res Rev. 2008;14:221–228. doi: 10.1002/ddrr.25. [DOI] [PubMed] [Google Scholar]
- Wess J, Nakajima K, Jain S. Novel designer receptors to probe GPCR signaling and physiology. Trends Pharmacol Sci. 2013;34:385–392. doi: 10.1016/j.tips.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN. The postnatal development of the visual cortex and the influence of the environment. In: TaL Frongsmyr J, editor. Nobel Lectures, Physiology or Medicine, 1981–1990. Singapore: World Scientific Publishing Company; 1981. [Google Scholar]
- Wilber AA, Lin GL, Wellman CL. Neonatal corticosterone administration impairs adult eyeblink conditioning and decreases glucocorticoid receptor expression in the cerebellar interpositus nucleus. Neuroscience. 2011;177:56–65. doi: 10.1016/j.neuroscience.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsey AJ, Sanders SJ, Li M, Dong S, Tebbenkamp AT, Muhle RA, Reilly SK, Lin L, Fertuzinhos S, Miller JA, Murtha MT, Bichsel C, Niu W, Cotney J, Ercan-Sencicek AG, Gockley J, Gupta AR, Han W, He X, Hoffman EJ, Klei L, Lei J, Liu W, Liu L, Lu C, Xu X, Zhu Y, Mane SM, Lein ES, Wei L, Noonan JP, Roeder K, Devlin B, Sestan N, State MW. Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell. 2013;155:997–1007. doi: 10.1016/j.cell.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf U, Rapoport MJ, Schweizer TA. Evaluating the affective component of the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2009;21:245–253. doi: 10.1176/jnp.2009.21.3.245. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998;2:338–347. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- Yang E-J, Lin EW, Hensch TK. Critical period for acoustic preference in mice. Proc Natl Acad Sci USA. 2012;109(Suppl 2):17213–17220. doi: 10.1073/pnas.1200705109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervas M, Blaess S, Joyner AL. Classical embryological studies and modern genetic analysis of midbrain and cerebellum development. Curr Top Dev Biol. 2005;69:101–138. doi: 10.1016/S0070-2153(05)69005-9. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]