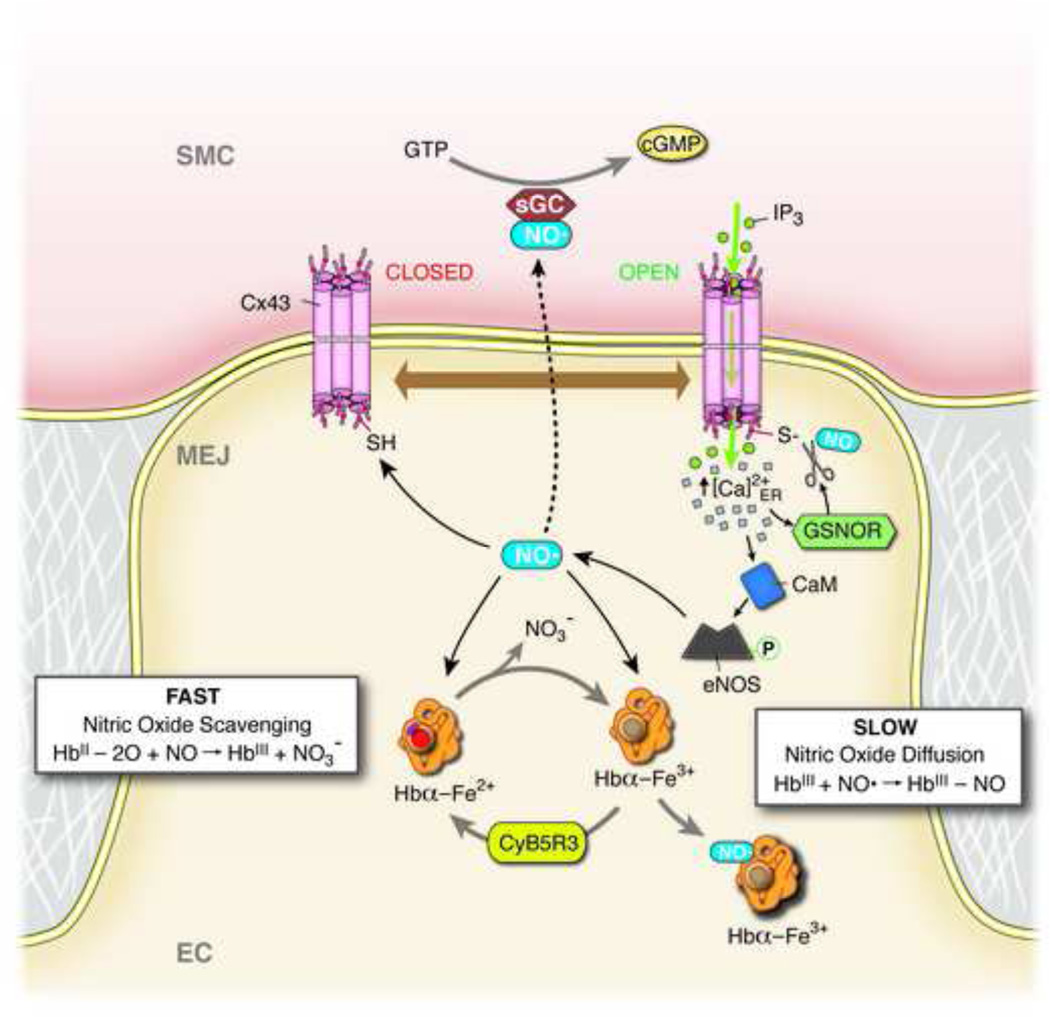
Figure 2. Schematic of NO regulation at the MEJ.
Negative feedback following α1-adrenergic receptor induced vasoconstriction at the myoendothelial junction (MEJ) of an endothelial cell (EC). α1-adrenergic receptor agonists cause an increase in smooth muscle cell (VSMC) cytosolic inositol 1,4,5-trisphosphate (IP3), which travels down its concentration gradient through open (i.e. S-nitrosylated) connexin 43 (Cx43) channels. IP3 binds to localized IPs-receptor 1 on the endoplasmic reticulum (not shown), which causes localized calcium (Ca2+) release and an increase in cytosolic Ca2+ concentration at the MEJ. This increase in cytosolic Ca2+ facilitates activation of S-nitrosoglutathione reductase (GSNOR), which denitrosylates and closes Cx43 channels, making them impermeable to IP3. The increase in cytosolic Ca2+ also activates calmodulin (CaM) through binding-induced conformational changes. The Ca2+/CaM complex binds to endothelial nitric oxide synthase (eNOS), facilitating its phosphorylation, activation, and production of nitric oxide (NO). Some of this newly-produced NO will diffuse into VSMC, where it binds to and activates soluble guanylyl cyclase (sGC), facilitating the conversion of GTP to cGMP, which ultimately leads to a reduction in constriction. The newly-produced NO may also facilitate opening of Cx43 channels through S-nitrosylation. Finally, excess NO may be scavenged by free hemoglobin alpha (Hbα) at the MEJ through reaction with oxyhemoglobin alpha (fast), or NO may be chelated through reaction with methemoglobin alpha (slow). Cytochrome B5 reductase (CyB5R3) converts methemoglobin alpha to Hbα, which readily binds oxygen3,6,21–23.