FIG 1.
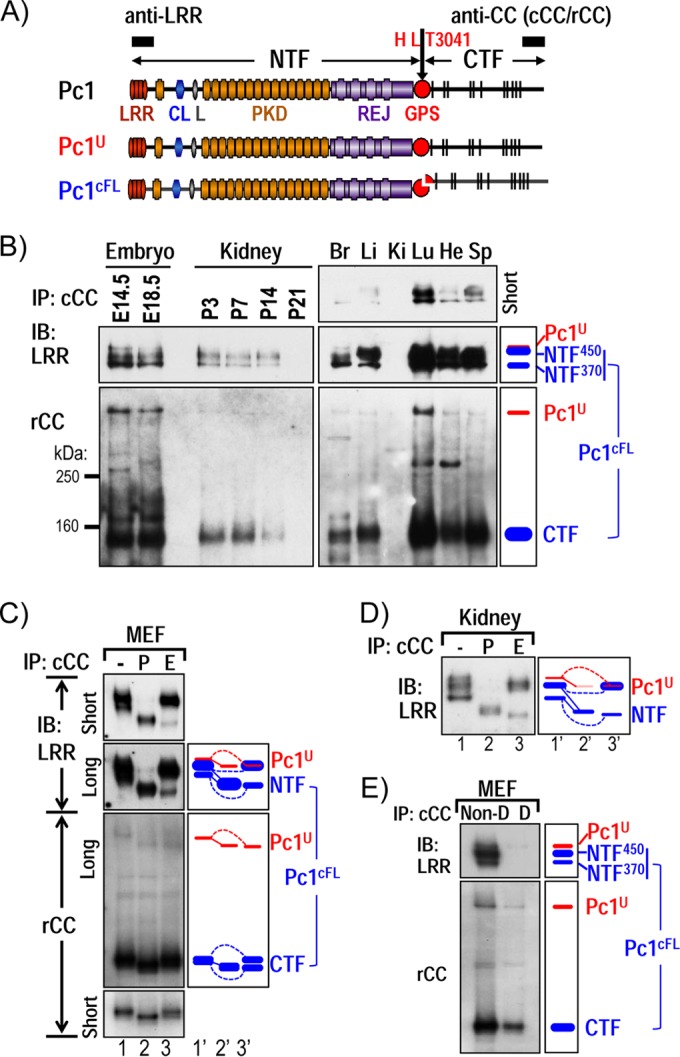
Characterization of endogenous Pc1U and Pc1cFL molecules in normal mouse tissues. (A) Schematic structure of mouse polycystin-1 (Pc1). LRR, leucine-rich repeat; CL, C-type lectin; L, LDL-A; PKD, polycystic kidney disease repeats; REJ, receptor for egg jelly; GPS, G-protein-coupled receptor proteolytic site. Pc1 cleavage occurs at HL↓T3041 site in the GPS motif, resulting in NTF and CTF fragments. Epitope positions of anti-LRR and anti-CC (chicken, cCC; rabbit, rCC) are shown by black boxes. The uncleaved full-length Pc1U (red) and the full-length cleaved Pc1cFL (blue) are schematized. The color code is maintained throughout the figures. (B) Endogenous Pc1 products were analyzed by immunoprecipitation (IP) with anti-cCC from wild-type (WT) mouse embryos (E14.5 and E18.5), kidneys (P3 to P21), and adult (2-month-old) tissues (Br, brain; Li, liver; Ki, kidney; Lu, lung; He, heart; Sp, spleen) and detected by immunoblotting (IB) with anti-LRR (upper panel) and anti-rCC (lower panel). The schematic diagram (right panel) provides an identification guide. (C) N-glycosylation modification of endogenous Pc1 from WT MEFs was monitored by IB on anti-cCC immunoprecipitates, either untreated (−) or treated with PNGase F (P) or endo-H (E). Pc1 products were detected with anti-LRR and anti-rCC from different exposures. The exclusive endo-H sensitivity of Pc1U contrasts with the partial endo-H sensitivity of Pc1cFL. Note that endo-H-deglycosylated Pc1U overlapped with the intense endo-H-resistant NTF450 band (lane 3). A schematic diagram provides an identification guide. (D) N-glycosylation modification of endogenous Pc1 from WT kidneys at P5 was analyzed as described for panel C and detected by IB with anti-LRR. Endogenous Pc1U is endo-H sensitive, whereas the Pc1 NTF subunit is both endo-H resistant and sensitive. (E) Noncovalent association of Pc1cFL subunits. MEF lysates were subjected to IP with anti-cCC under either nondenaturing conditions with 0.5% Triton X-100 (Non-D) or denaturing conditions with detergent SDS (0.1%; D), followed by IB with anti-LRR or anti-rCC. The NTF subunit was coprecipitated by the CTF subunit only under nondenaturing conditions. The schematic diagram provides an identification guide.
