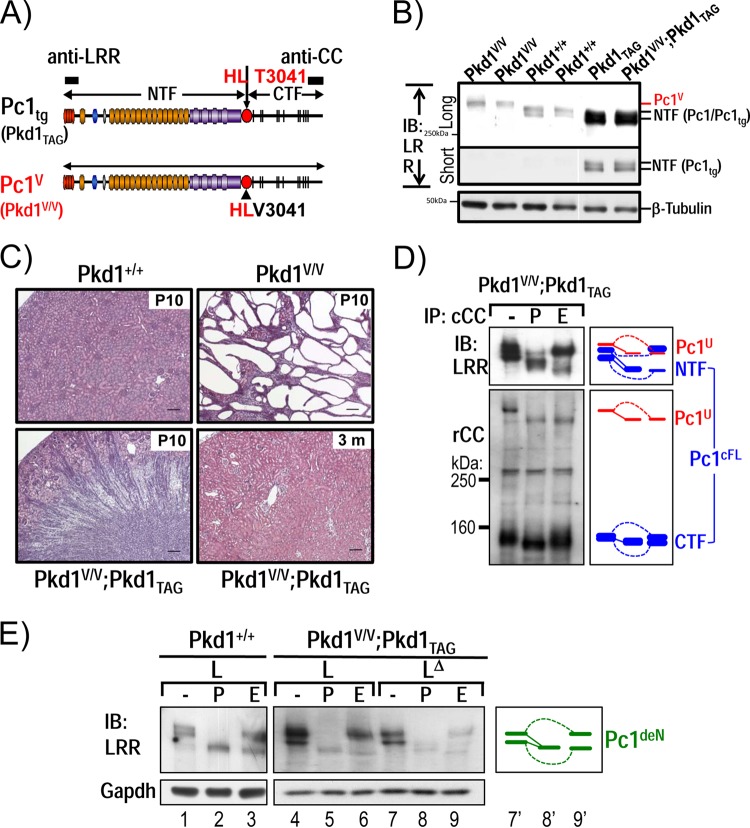
FIG 7.
Functional complementation of Pkd1V/V by Pkd1-BAC transgenic mice. (A) Schematic diagram of Pc1tg (Pkd1TAG) and Pc1V (Pkd1V/V). The epitopes recognized by anti-LRR and anti-CC are indicated as black boxes. (B) Protein expression of P10 kidneys from Pkd1+/+, Pkd1TAG, Pkd1V/V, and Pkd1V/V; Pkd1TAG mice were analyzed by IB using anti-LRR. Pc1 expression in Pkd1TAG and Pkd1V/V; Pkd1TAG mice was increased, and Pc1 migrated as a doublet, like endogenous Pc1. β-Tubulin served as a loading control. (C) Kidney histology (H&E staining) of Pkd1+/+, Pkd1V/V, and Pkd1V/V; Pkd1TAG mice. Pkd1V/V; Pkd1TAG mice showed complete rescue of the Pkd1V/V renal phenotype, similar to the WT controls at P10 and 3 months of age. Scale bar, 100 μm. (D) N-glycosylation status of Pc1 from Pkd1V/V; Pkd1TAG P10 kidneys was monitored by IB on anti-cCC immunoprecipitates, either untreated (−) or treated with PNGase F (P) or endo-H (E). Pc1 products were detected with anti-LRR and anti-rCC as indicated. Pc1cFL and Pc1U patterns in Pkd1V/V; Pkd1TAG kidneys are identical to those of the endogenous Pc1 in WT kidneys shown in Fig. 1D. The schematic diagram indicates different Pc1 forms. (E) Pc1 N-glycosylation status of wild-type Pkd1+/+ and Pkd1V/V; Pkd1TAG P10 kidneys was analyzed using total lysate (L) and immunodepleted lysate (LΔ) by IB with anti-LRR following deglycosylation. Pkd1V/V; Pkd1TAG kidneys produce both endo-H-resistant and -sensitive Pc1deN forms as in WT kidneys (left panel). The schematic diagram provides an identification guide.