FIG 2.
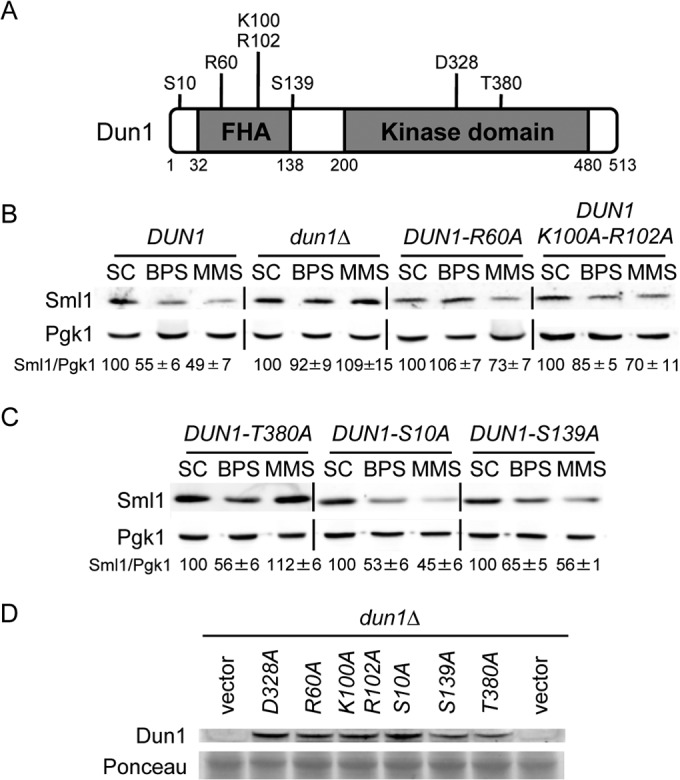
Structure-function analysis of Dun1 protein domains required for Sml1 protein decline in response to iron deficiency. (A) Schematic representation of the most relevant Dun1 domains and amino acid residues. The numbers indicate the amino acid positions. (B) The integrity of the Dun1 FHA domain is essential for the drop in Sml1 protein caused by BPS treatment. Yeast dun1Δ (SPY350) cells transformed with plasmid p413-DUN1(EcoRI) (DUN1), pRS413 (dun1Δ), p413-DUN1-R60A (DUN1-R60A), or p413-DUN1-K100A-R102A (DUN1-K100A-R102A) were grown as described in the legend to Fig. 1. (C) Sml1 protein levels in yeast cells lacking specific Dun1 phosphorylation sites. Yeast dun1Δ (SPY350) cells transformed with plasmid p413-DUN1-T380A (DUN1-T380A), p413-DUN1-S10A (DUN1-S10A), or p413-DUN1-S139A (DUN1-S139A) were grown as described in the legend to Fig. 1. For each transformant in panels B and C, quantitation of Sml1/Pgk1 protein levels under BPS and MMS conditions is relative to the corresponding values obtained in SC medium. A representative image and the average and the standard deviation of three independent biological replicates are shown for each transformant. (D) Mutant Dun1 protein levels under low-iron conditions. Yeast dun1Δ (SPY350) cells transformed with plasmid pRS416, pMH62 (DUN1-D328A) p413-DUN1-R60A (R60A), p413-DUN1-K100A,R102A (K100A-R102A), p413-DUN1-S10A (S10A), p413-DUN1-S139A (S139A), or p413-DUN1-T380A (T380A) were grown at 30°C for 6 h in SC medium with 100 μM BPS. Dun1 protein levels were determined by immunoblotting with an anti-Dun1 antibody, and Ponceau staining was used as a loading control.
