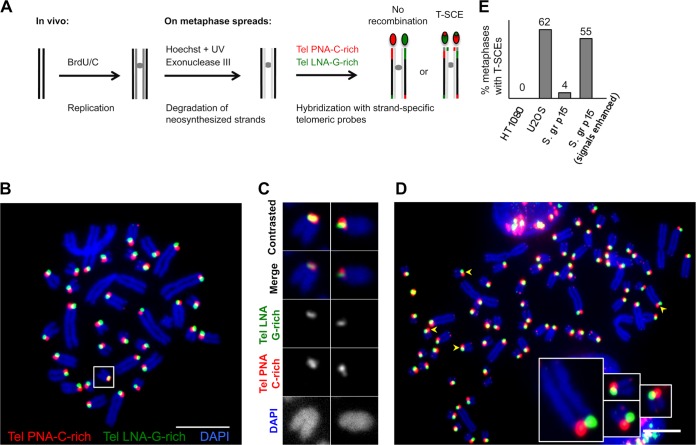
FIG 5.
Recombination at telomeres in S. granarius. (A) CO-FISH approach to reveal T-SCEs. After the removal of newly synthesized strands, a C-rich probe will exclusively detect the parental G-rich strand (one-color CO-FISH). If an exchange has taken place after replication, two signals instead of one will be detected at the chromosome extremity. In two-color CO-FISH, strand-specific C-rich and G-rich telomeric probes are used, and T-SCEs are detected as mixed red-green signals. (B) CO-FISH using two strand-specific telomeric probes: TelPNA-C-rich-Cy3 (red) and Tel-LNA-G-rich-FAM (green). Chromosomes were counterstained with DAPI (blue). Only long telomeres present on short acrocentric arms were analyzed. Most extremities show one single green or red robust signal per chromatid. The box indicates a chromosome with mixed signals, indicating a T-SCE. Affected chromosomes varied from metaphase to metaphase. Quantifications of different experiments using one- or two-color CO-FISH are presented in panel E and Table 2. (C) Examples of T-SCEs detected in S. granarius early-passage fibroblasts (p15). (D) Signal enhancement allows the detection of potential highly asymmetric exchanges (very weak green signals colocalizing with the strong red signal, and vice versa [arrowheads]). Enlarged examples are presented. The segregated CO-FISH analysis presented in Fig. 6 indicates that such colocalizations correspond to bona fide T-SCEs. Bars, 10 μm. (E) Quantification of T-SCEs in S. granarius early-passage (S. gr p15) fibroblasts relative to U2OS/ALT and HT1080/TEL+ human cancer cell lines (n = 30 metaphase spreads for each condition). The frequency of metaphase chromosomes carrying T-SCEs when only “robust” fluorescence signals are taken into account appears to be low in S. granarius fibroblasts. However, when T-SCEs are searched upon enhancement of signals, the frequency is much higher. The fact that these are bone fide T-SCEs was confirmed by segregated CO-FISH analysis (Fig. 6).