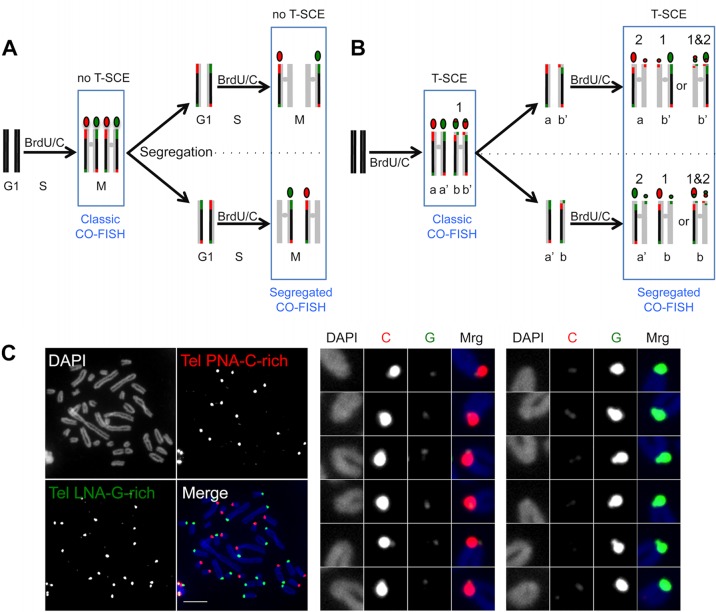
FIG 6.
(A) BrdU/C incorporation during two cell cycles prior to the CO-FISH procedure, using strand-specific telomeric probes, results in segregation of the unsubstituted G- and C-rich strands into different chromosomes, such that during the second M phase, every chromosome extremity will be stained, after the CO-FISH procedure, with only one probe, either red (TelPNA-C-rich-Cy3) or green (TelLNA-G-rich-FAM). (B) If a T-SCE occurs during the first cell cycle, the two unsubstituted strands will cosegregate during the first mitosis and will be detected on different sister chromatids of the same chromosome during the second M phase. The CO-FISH procedure will then reveal one red and one green signal on the same chromosome extremity (marked 1). If a T-SCE occurs during the second cell cycle, this exchange will result in same-color doublets, either red or green (marked 2). However, if a second exchange affects an extremity that had already undergone T-SCE during the first cell cycle, doublets will be of both colors (marked 1&2). (C) Two-color segregated CO-FISH in S. granarius. Highly asymmetric two-color doublets are frequently detected in S. granarius fibroblasts (p15). Examples of such T-SCEs are enlarged and color decomposed on the right. The TelPNA-C-rich-Cy3 probe is more efficient than the TelLNA-G-rich-FAM probe for detecting small doublets. Chromosomes were counterstained with DAPI (blue). Bar, 10 μm.