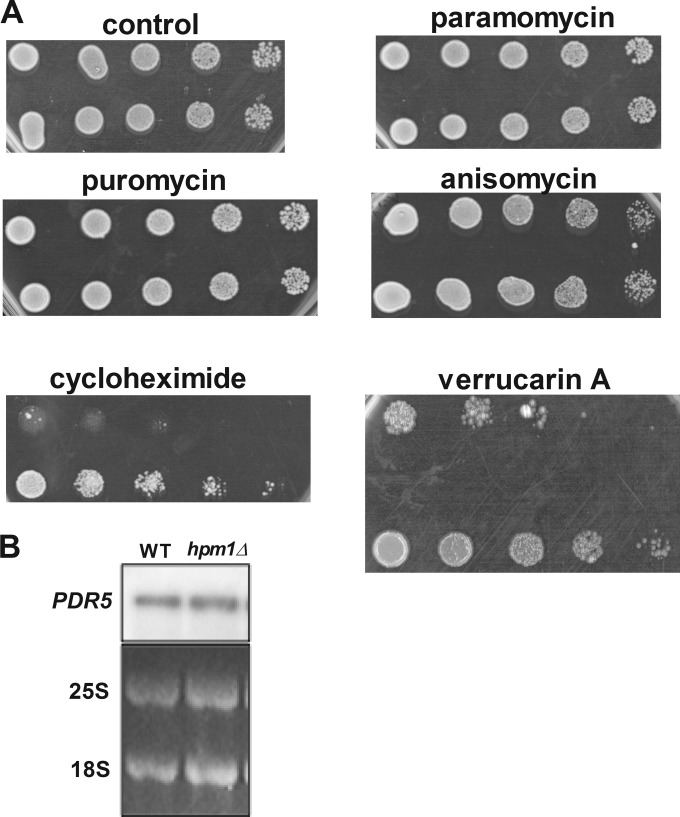
FIG 6.
Lack of HPM1 results in greatly increased resistance to the ribosome-targeting drugs cycloheximide and verrucarin A. (A) Dilution spot assays were done with mid-log-phase yeast cells (OD600 of 0.5) grown in YPD medium at 30°C in a rotary shaker for 2 generations from an overnight culture grown in YPD at 30°C. Cells were serially diluted 5-fold on YPD agar plates in the presence or absence of the ribosome-targeting antibiotics puromycin (50 μg/ml), cycloheximide (500 ng/ml), paromomycin (50 μg/ml), anisomycin (5 μg/ml), and verrucarin A (2 μg/ml) and incubated at 30°C for 2 days (control, puromycin, anisomycin, and paromomycin), 3 days (verrucarin A), or 5 days (cycloheximide). WT and hpm1Δ mutant cells containing the empty pUG35 vector were also spotted onto SD-Ura-Met and incubated for 3 days. (B) Northern blot analysis of mRNA expressed from the PDR5 gene encoding the multidrug exporter PDR5. The 25S and 18S blots are from the ethidium bromide fluorescence of the gel prior to transfer.