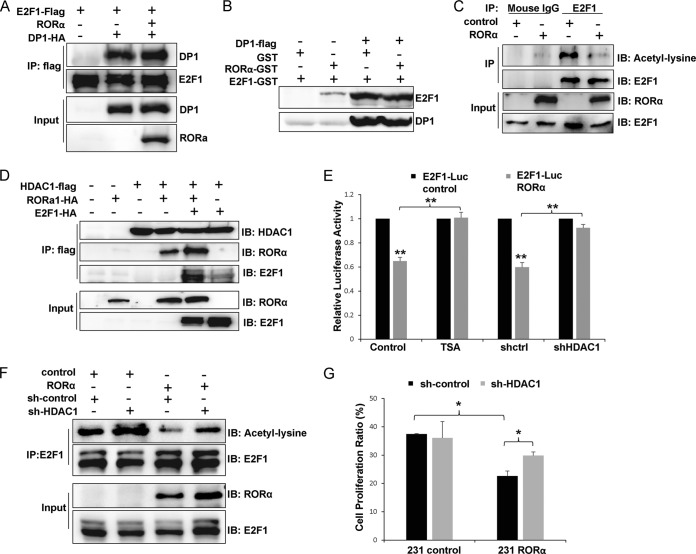
FIG 4.
RORα reduces E2F1 acetylation by recruiting HDAC1. (A) Coimmunoprecipitation experiments were performed with HEK293 cells cotransfected with RORα, Flag-tagged E2F1, and HA-tagged DP1. (B) A binding assay showed that binding of Flag-tagged DP1 to GST-tagged E2F1 was not inhibited by RORα. (C) Lysine acetylation of endogenous E2F1 was inhibited by RORα in MDA-MB 231 cells. (D) RORα and E2F1 both interacted with HDAC1 and were examined by immunoprecipitation experiments performed with HEK293 cells transfected with HDAC1, RORα, and E2F1. (E) A luciferase reporter assay showed that inhibition of HDAC1 activity (TSA treatment or knockdown of HDAC1 by shHDAC1-containing lentivirus) rescued E2F1 activity in RORα-expressing MDA-MB 231 cells (n = 3; **, P < 0.01). (F) Immunoprecipitation experiment results showed that knockdown of HDAC1 rescued E2F1 acetylation in RORα-expressing MDA-MB 231 cells. (G) EdU staining results showed that silencing HDAC1 partially rescued cell proliferation in RORα-expressing MDA-MB 231 cells (n = 3; *, P < 0.05).