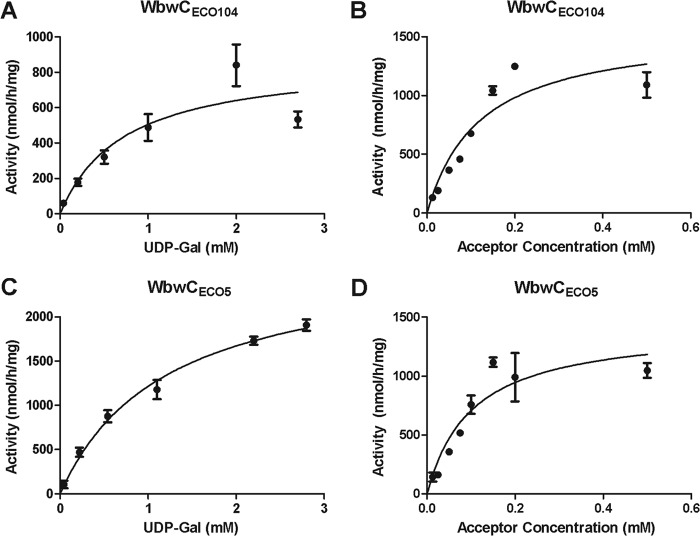
FIG 2.
Kinetics of WbwCECO104 and WbwCECO5 reactions. The standard assay as described in Materials and Methods was used to measure Gal transfer by purified WbwC enzymes. (A) WbwCECO104 reaction with acceptor 8 (0.25 mM) as a function of UDP-Gal concentration. The apparent Km for UDP-Gal was 0.73 mM with an apparent Vmax of 0.87 μmol/h/mg protein. (B) WbwCECO104 reaction as a function of acceptor 8 concentration. UDP-Gal concentration was 2.2 mM. The apparent Km for 8 was 0.12 mM with an apparent Vmax of 1.57 μmol/h/mg protein. (C) WbwCECO5 reaction with 0.25 mM acceptor 8 as a function of UDP-Gal concentration. The apparent Km for UDP-Gal was 1.20 mM with an apparent Vmax of 2.67 μmol/h/mg protein. (D) WbwCECO5 reaction as a function of acceptor 8. UDP-Gal donor concentration was 1.09 mM. The apparent Km for acceptor 8 was 0.10 mM with an apparent Vmax of 1.42 μmol/h/mg protein. Substrate inhibition was apparent at high acceptor concentration (not shown). All results were analyzed by regression analysis with GraphPad Prism.