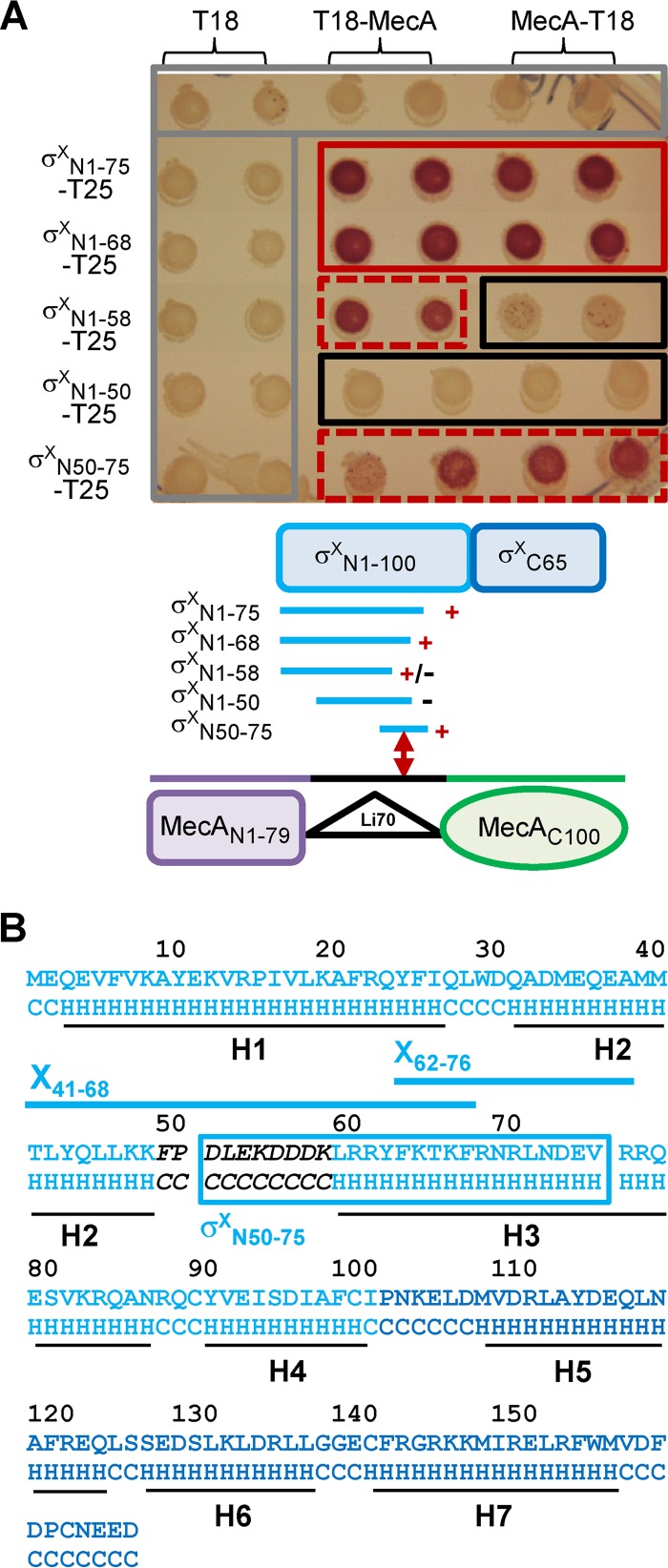
FIG 3.
Interactions between MecA and truncated N-domain variants of σX, evaluated by B2H assays. (A) Top, matrices of B2H interactions between full-length MecA and truncated N-domain variants of σX on MacConkey plates. The color code for rectangles is the same as in Fig. 2. Bottom, organization of MecA and σX domains and summary of interactions. +, +/−, and − indicate positive, weak, and negative interactions, respectively. (B) σX sequence and predicted secondary structure (LOMETS server [http://zhanglab.ccmb.med.umich.edu/LOMETS/]) with α-helixes H1 to H7 (black lines). The predicted secondary structure is indicated below the protein sequence. H, α-helix; C, coil. Light and dark blue sequences correspond to the N and C domains of σX, respectively. X41-68 and X62-76 peptides and their position are mapped with blue lines. σXN50-75, which interacts with MecA in B2H assays, is indicated by a blue box. The sequence of the predicted surface-exposed loop is in black italic.