FIG 9.
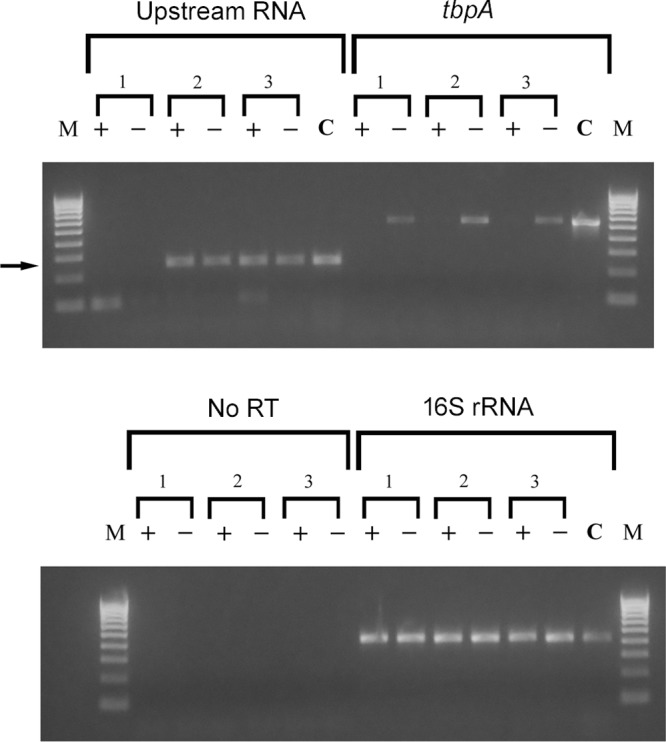
RT-PCR experiment demonstrating that Ω insertion into R2 (−447 relative to the transcriptional start site) prevents expression of the upstream RNA species. Cultures of MCV113 (1), MCV114 (2), and wild-type FA19 (3) were grown under iron-replete (+) and iron-depleted (−) conditions. RNA was isolated from each culture and subjected to reverse transcription. oVCU735C was used to reverse transcribe the upstream RNA, and random hexamers were used to reverse transcribe tbpA and 16S rRNA. A negative control in which no reverse transcriptase was added, along with random hexamers, was also conducted (no RT). For PCRs, the following oligonucleotides were utilized: oVCU735C and oVCU199C to amplify the upstream RNA, oVCU186 and oVCU187 to amplify tbpA, and oVCU110 and oVCU111 to amplify 16S rRNA and no-RT reactions. Chromosomal DNA from FA19 (C) also was amplified with the same primer sets to demonstrate the wild-type size of each amplicon. Molecular mass markers are shown on the left and right of each ethidium bromide-stained gel. Markers (M) range from 100 bp to 1,000 bp and increase in increments of 100 bp. The arrow indicates the position of the amplicon that was amplified with primers oVCU735C and oVCU199C, corresponding to the upstream RNA.
