Abstract
In most eukaryotic cells, the respiratory chain NADH dehydrogenase (Complex I) is a multimeric enzyme under dual (nuclear and mitochondrial) genetic control. Several genes encoding subunits of this enzyme have been identified in the mitochondrial genome from various organisms, but the functions of these subunits are in most part unknown. We describe here a human cell line in which the enzyme lacks the mtDNA-encoded subunit ND4 due to a frameshift mutation in the gene. In this cell line, the other mtDNA-encoded subunits fail to assemble, while at least some of the nuclear-encoded subunits involved in the redox reactions appear to be assembled normally. In fact, while there is a complete loss of NADH:Q1 oxidoreductase activity, the NADH:Fe(CN)6 oxidoreductase activity is normal. These observations provide the first clear evidence that the ND4 gene product is essential for Complex I activity and give some insights into the function and the structural relationship of this polypeptide to the rest of the enzyme. They are also significant for understanding the pathogenetic mechanism of the ND4 gene mutation associated with Leber's hereditary optic neuropathy.
Full text
PDF
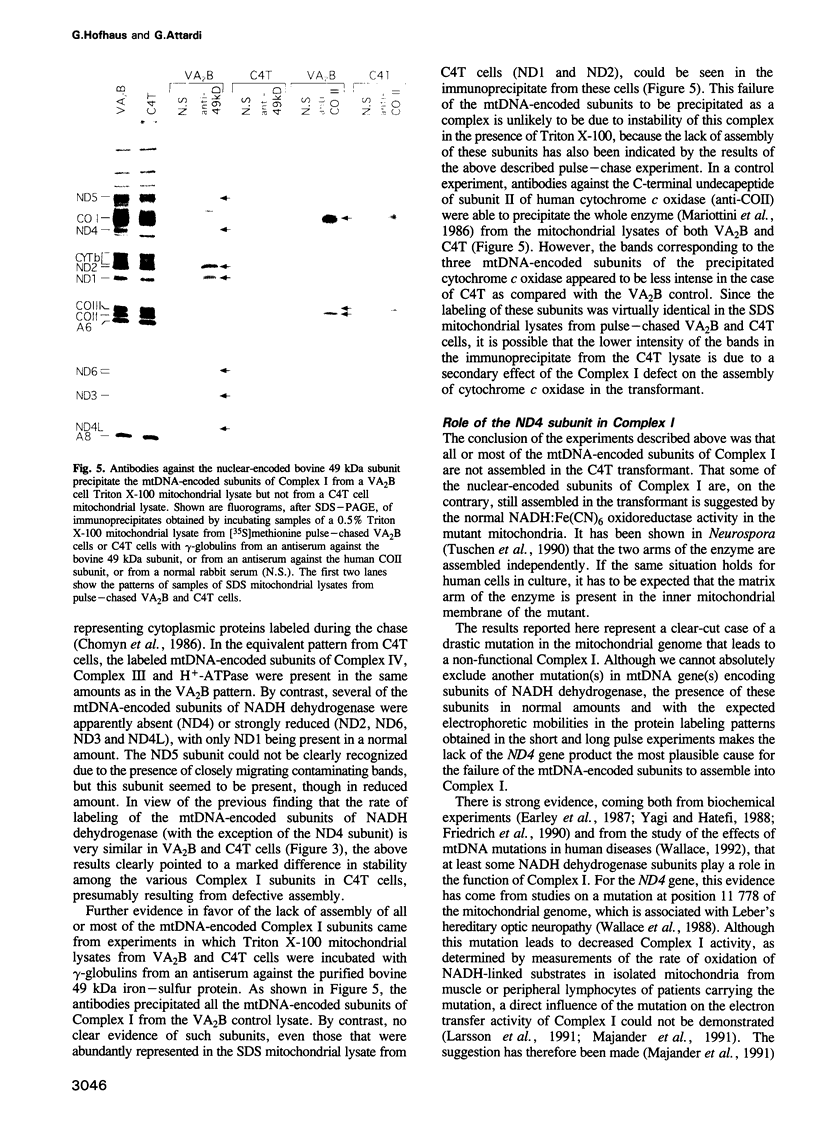


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Cleeter M. W., Ragan C. I., Riley M., Doolittle R. F., Attardi G. URF6, last unidentified reading frame of human mtDNA, codes for an NADH dehydrogenase subunit. Science. 1986 Oct 31;234(4776):614–618. doi: 10.1126/science.3764430. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Mariottini P., Cleeter M. W., Ragan C. I., Matsuno-Yagi A., Hatefi Y., Doolittle R. F., Attardi G. Six unidentified reading frames of human mitochondrial DNA encode components of the respiratory-chain NADH dehydrogenase. Nature. 1985 Apr 18;314(6012):592–597. doi: 10.1038/314592a0. [DOI] [PubMed] [Google Scholar]
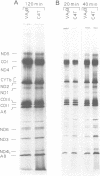
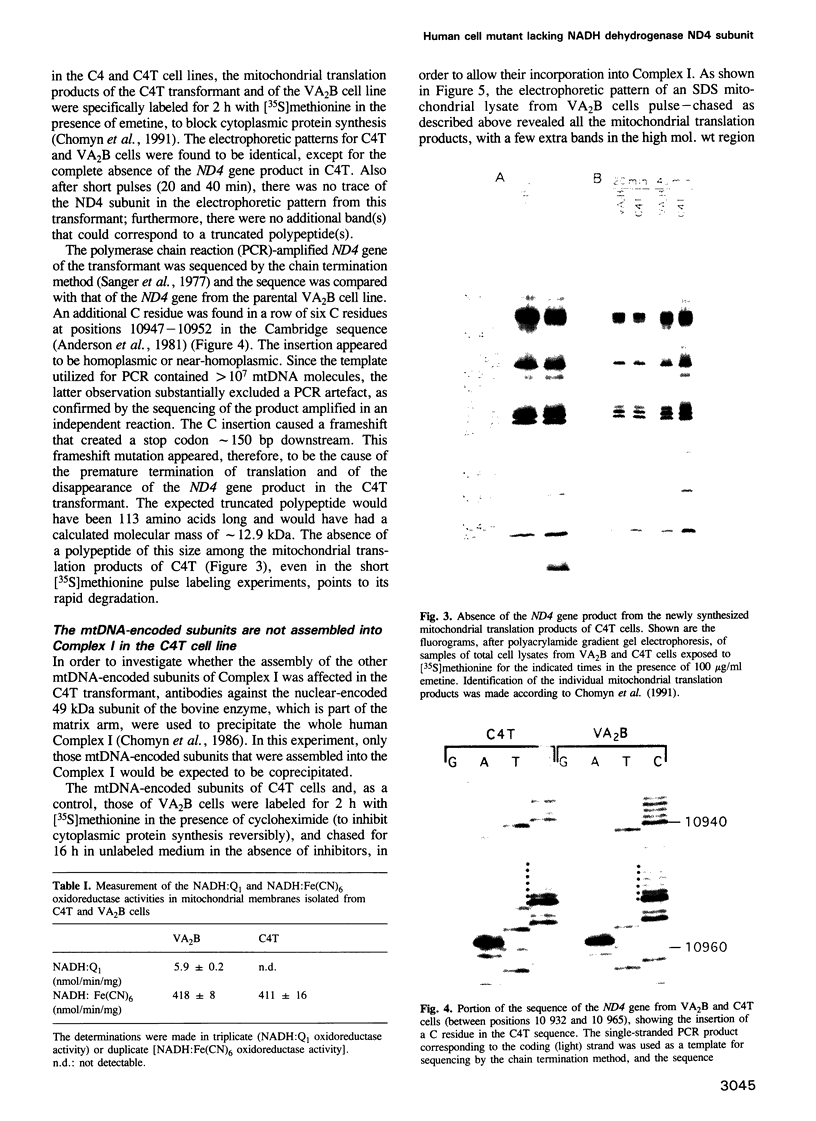
- Chomyn A., Meola G., Bresolin N., Lai S. T., Scarlato G., Attardi G. In vitro genetic transfer of protein synthesis and respiration defects to mitochondrial DNA-less cells with myopathy-patient mitochondria. Mol Cell Biol. 1991 Apr;11(4):2236–2244. doi: 10.1128/mcb.11.4.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley F. G., Patel S. D., Ragan I., Attardi G. Photolabelling of a mitochondrially encoded subunit of NADH dehydrogenase with [3H]dihydrorotenone. FEBS Lett. 1987 Jul 13;219(1):108–112. doi: 10.1016/0014-5793(87)81200-0. [DOI] [PubMed] [Google Scholar]
- Friedrich T., Strohdeicher M., Hofhaus G., Preis D., Sahm H., Weiss H. The same domain motif for ubiquinone reduction in mitochondrial or chloroplast NADH dehydrogenase and bacterial glucose dehydrogenase. FEBS Lett. 1990 Jun 4;265(1-2):37–40. doi: 10.1016/0014-5793(90)80878-m. [DOI] [PubMed] [Google Scholar]
- Galante Y. M., Hatefi Y. Purification and molecular and enzymic properties of mitochondrial NADH dehydrogenase. Arch Biochem Biophys. 1979 Feb;192(2):559–568. doi: 10.1016/0003-9861(79)90126-7. [DOI] [PubMed] [Google Scholar]
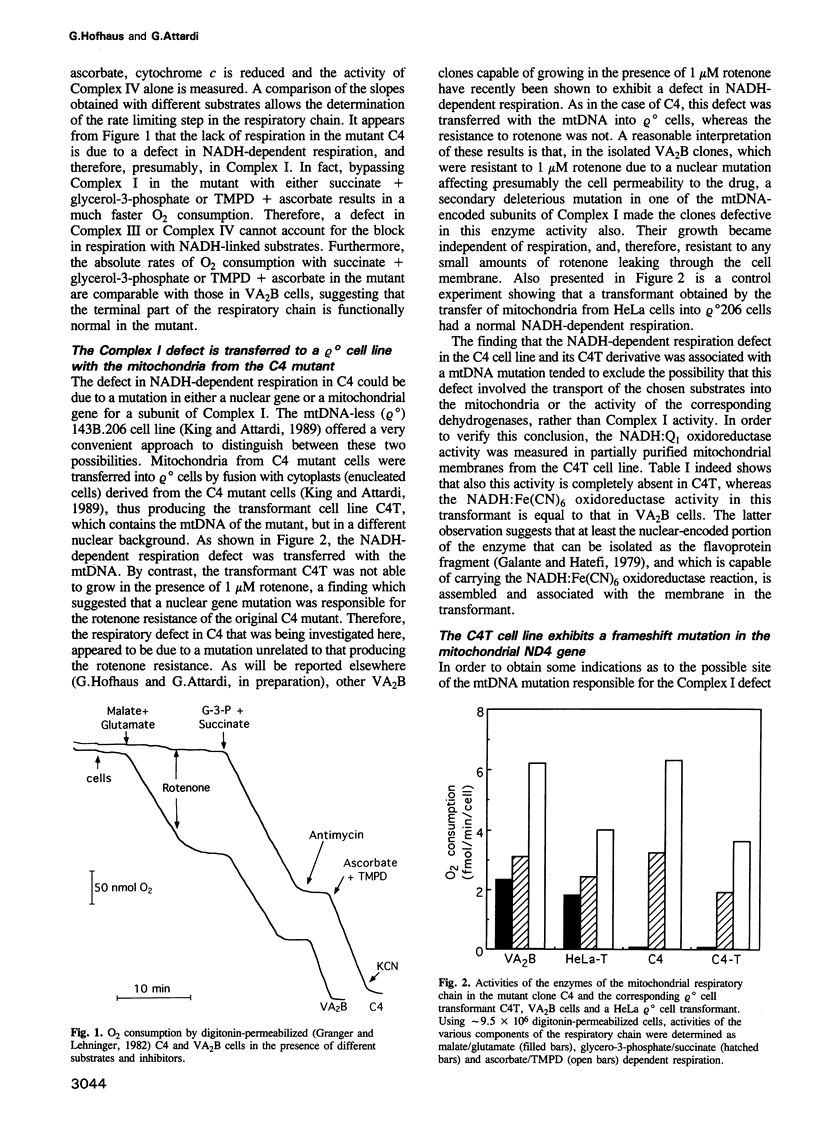
- Granger D. L., Lehninger A. L. Sites of inhibition of mitochondrial electron transport in macrophage-injured neoplastic cells. J Cell Biol. 1982 Nov;95(2 Pt 1):527–535. doi: 10.1083/jcb.95.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofhaus G., Weiss H., Leonard K. Electron microscopic analysis of the peripheral and membrane parts of mitochondrial NADH dehydrogenase (complex I). J Mol Biol. 1991 Oct 5;221(3):1027–1043. doi: 10.1016/0022-2836(91)80190-6. [DOI] [PubMed] [Google Scholar]
- King M. P., Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989 Oct 27;246(4929):500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- Majander A., Huoponen K., Savontaus M. L., Nikoskelainen E., Wikström M. Electron transfer properties of NADH:ubiquinone reductase in the ND1/3460 and the ND4/11778 mutations of the Leber hereditary optic neuroretinopathy (LHON). FEBS Lett. 1991 Nov 4;292(1-2):289–292. doi: 10.1016/0014-5793(91)80886-8. [DOI] [PubMed] [Google Scholar]
- Mariottini P., Chomyn A., Doolittle R. F., Attardi G. Antibodies against the COOH-terminal undecapeptide of subunit II, but not those against the NH2-terminal decapeptide, immunoprecipitate the whole human cytochrome c oxidase complex. J Biol Chem. 1986 Mar 5;261(7):3355–3362. [PubMed] [Google Scholar]
- Mitchell C. H., England J. M., Attardi G. Isolation of chloramphenicol-resistant variants from a human cell line. Somatic Cell Genet. 1975 Jul;1(3):215–234. doi: 10.1007/BF01538447. [DOI] [PubMed] [Google Scholar]
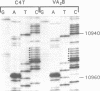
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumegi B., Srere P. A. Complex I binds several mitochondrial NAD-coupled dehydrogenases. J Biol Chem. 1984 Dec 25;259(24):15040–15045. [PubMed] [Google Scholar]
- Tuschen G., Sackmann U., Nehls U., Haiker H., Buse G., Weiss H. Assembly of NADH: ubiquinone reductase (complex I) in Neurospora mitochondria. Independent pathways of nuclear-encoded and mitochondrially encoded subunits. J Mol Biol. 1990 Jun 20;213(4):845–857. doi: 10.1016/S0022-2836(05)80268-2. [DOI] [PubMed] [Google Scholar]
- Walker J. E. The NADH:ubiquinone oxidoreductase (complex I) of respiratory chains. Q Rev Biophys. 1992 Aug;25(3):253–324. doi: 10.1017/s003358350000425x. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Diseases of the mitochondrial DNA. Annu Rev Biochem. 1992;61:1175–1212. doi: 10.1146/annurev.bi.61.070192.005523. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Singh G., Lott M. T., Hodge J. A., Schurr T. G., Lezza A. M., Elsas L. J., 2nd, Nikoskelainen E. K. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988 Dec 9;242(4884):1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- Weiss H., Friedrich T., Hofhaus G., Preis D. The respiratory-chain NADH dehydrogenase (complex I) of mitochondria. Eur J Biochem. 1991 May 8;197(3):563–576. doi: 10.1111/j.1432-1033.1991.tb15945.x. [DOI] [PubMed] [Google Scholar]
- Yagi T., Hatefi Y. Identification of the dicyclohexylcarbodiimide-binding subunit of NADH-ubiquinone oxidoreductase (Complex I). J Biol Chem. 1988 Nov 5;263(31):16150–16155. [PubMed] [Google Scholar]