FIG 2.
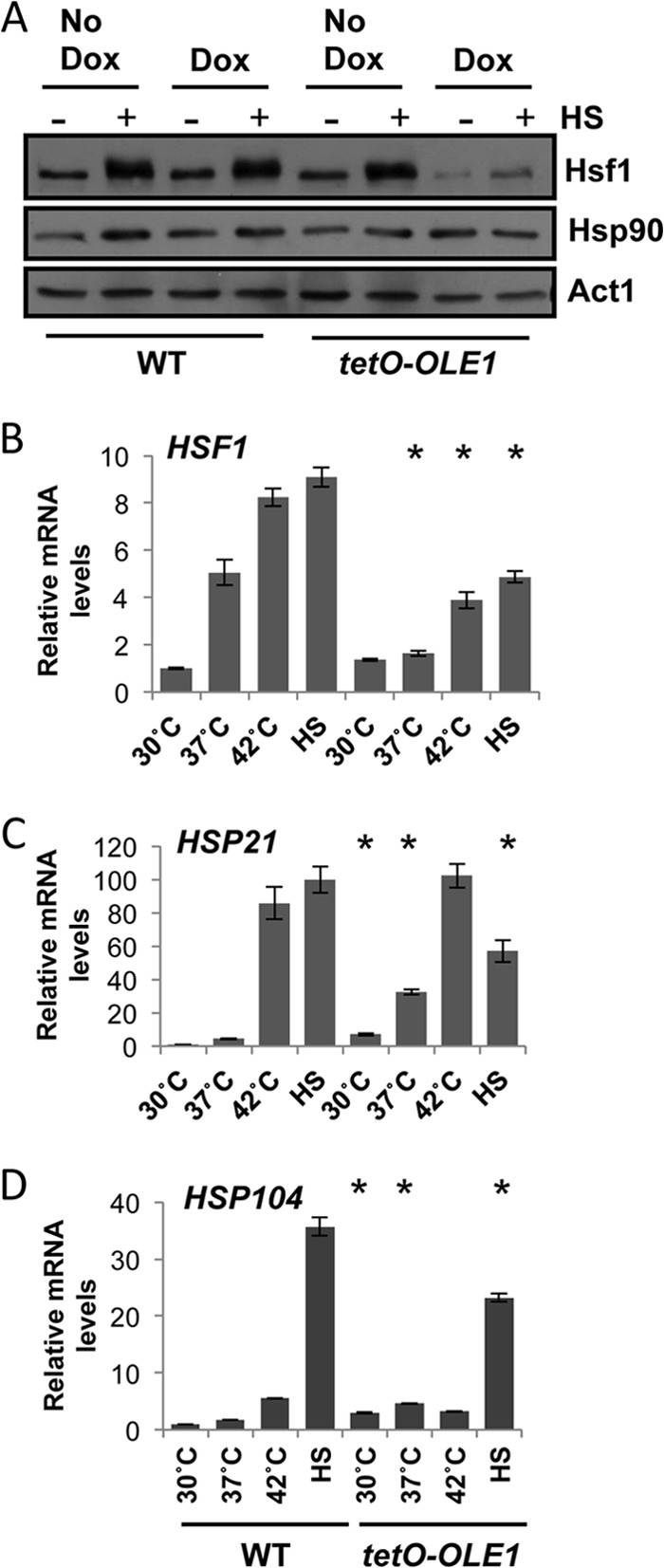
Depletion of Ole1 inhibits full activation of Hsf1, leading to a loss in HSP expression during high-temperature growth and heat shock. (A) Phosphorylation of C. albicans Hsf1 during a 30°C to 42°C heat shock, revealed by Western analysis. Exponentially growing WT (wild type, CaLC2993 [Table 1]) and tetO-OLE1/ole1Δ cells (CaLC3032) were treated or not with1 μg/ml of doxycycline for 4 h and subjected to a 15-min 30°C to 42°C heat shock. Protein was extracted immediately, and Hsf1 phosphorylation was monitored using an anti-TAP antibody recognizing HSF1-TAP. Membranes were stripped and reprobed for Hsp90, and actin served as an internal loading control. (B to D) Expression of HSF1 (B), HSP21 (C), and HSP104 (D) during growth at 30°C, 37°C, or 42°C or after a 15-min 30°C to 42°C heat shock (HS), as determined by qRT-PCR of the corresponding transcripts relative to the internal ACT1 mRNA control. *, P < 0.05 compared to the value for the wild type at the corresponding temperature (Student t test).
