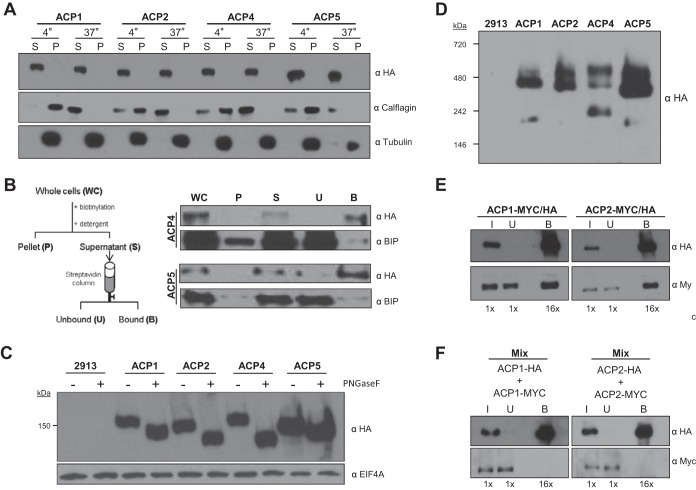
FIG 2.
Trypanosomal adenylate cyclases are glycosylated surface proteins that dimerize. (A) Western blot analysis of the proteins in the soluble (S) and insoluble pellet (P) fraction from cells extracted with Triton X-100 at the indicated temperature (4° or 37°C). Protein samples from cells expressing the indicated HA-tagged ACP (ACP1, ACP2, ACP4, or ACP5) were probed with antibodies against HA, calflagin, and tubulin. (B) Cells expressing HA-tagged ACP4 or ACP5 were surface biotinylated, and protein extracts harvested as indicated in the schematic were subjected to Western blot analysis with antibodies against HA or BiP. (C) Whole-cell lysates were prepared from 2913 control cells or cells expressing HA-tagged ACP1, ACP2, ACP4, or ACP5. Samples were untreated (−) or treated (+) with PNGase F and then subjected to Western blot analysis with antibodies against HA or EIF4AI. (D) Whole-cell lysates prepared from 2913 control cells or cells expressing HA-tagged ACP1, ACP2, ACP4, or ACP5 were separated by Blue Native gel electrophoresis and then transferred to a PVDF membrane and probed with anti-HA antibodies. (E) Cells with two tagged alleles of ACP1 (ACP1-Myc/HA) or two tagged alleles of ACP2 (ACP2-Myc/HA) were used for coimmunoprecipitation to test for homodimerization. In test samples, one allele of the indicated AC gene contains an HA tag and the other allele has a Myc tag. Samples were immunoprecipitated with anti-HA antibody, and input (I), unbound (U), and bound (B) fractions were probed in Western blots with anti-HA or anti-Myc antibody. As a negative control, ACP1-Myc lacks any HA tag and is not precipitated by anti-HA antibody (see Fig. S2 in the supplemental material). The numbers below each lane indicate the relative number of cell equivalents analyzed. (F) Cells expressing ACP1-HA were mixed with cells expressing ACP1-Myc, and the cells in the mixture were lysed and immunoprecipitated using anti-HA antibody. Input, unbound, and bound fractions were probed in Western blots with anti-HA or anti-Myc antibody.