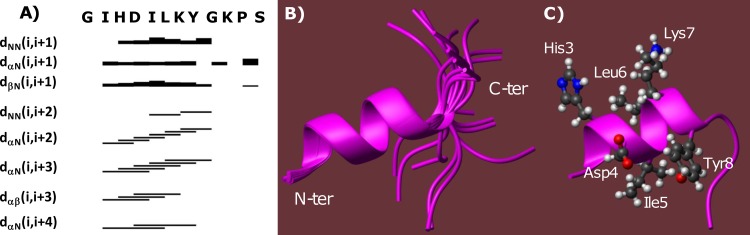
FIG 2.
(A) NOE intensity pattern. The myxinidin amino acid sequence is given at the top. Sequential and medium-range NOEs are represented by bars connecting different residues; the thickness of the lines reflects the corresponding NOE intensities. NOE connectivities involving the HN, Hα, and Hβ of residue i and amide protons of residue i + x are represented by dNN(i,i + x), dαN(i,i + x), and dβN(i,i + x), respectively, whereas dαβ(i,i + 3) denotes a NOE cross-peak between the Hα and Hβ protons of the i and i + 3 residues, respectively. (B) Overlay on the backbone atoms (residues 2 to 9) of 20 myxinidin NMR conformers. N-ter, N terminus; C-ter, C terminus. (C) Ribbon representation of the best CYANA structure (i.e., the one with the lowest target function: n.1); 142 distance constraints (54 intraresidue, 62 short-, and 26 medium-range) and 42 angle constraints were incorporated in the final structure calculation.