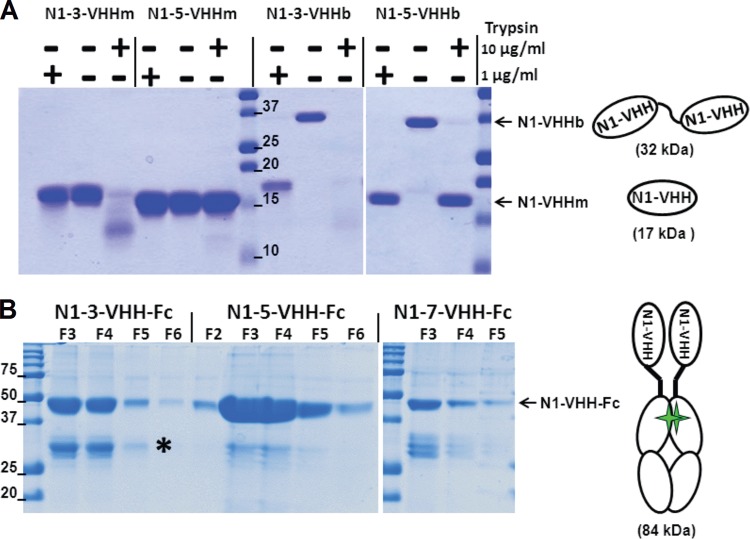
FIG 2.
N1-VHH monovalent and bivalent formats. (A) The llama IgG2c-derived hinge is sensitive to trypsin. Shown is Coomassie-stained reducing SDS-PAGE of purified N1-3-VHHm, N1-5-VHHm, N1-3-VHHb, and N1-5-VHHb incubated for 60 min at 37°C in the presence or absence of trypsin at 1 or 10 μg/ml. The N1-VHHb molecules migrate at about 32 kDa, and N1-VHHm and the cleavage products of N1-VHHb migrate as bands of about 17 kDa. The diagrams on the right depict N1-VHHm and N1-VHHb (consisting of two N1-VHHm moieties in tandem, linked by a llama IgG2c hinge of 17 amino acid residues). (B) Coomassie-stained reducing SDS-PAGE of the fractions eluted from a protein G column purification step with seed extracts of transgenic A. thaliana T3 plants expressing the N1-VHH-Fc proteins indicated. The N1-VHH-Fc constructs migrate at about 42 kDa. A degradation product (*) of about 28 kDa, corresponding to the Fc moiety only, is visible. The diagram on the right shows an N1-VHH-Fc molecule consisting of two N1-VHHm moieties fused to mouse IgG2a Fc, dimerized through a disulfide bond (green stars). The values beside the lanes are molecular sizes in kilodaltons.