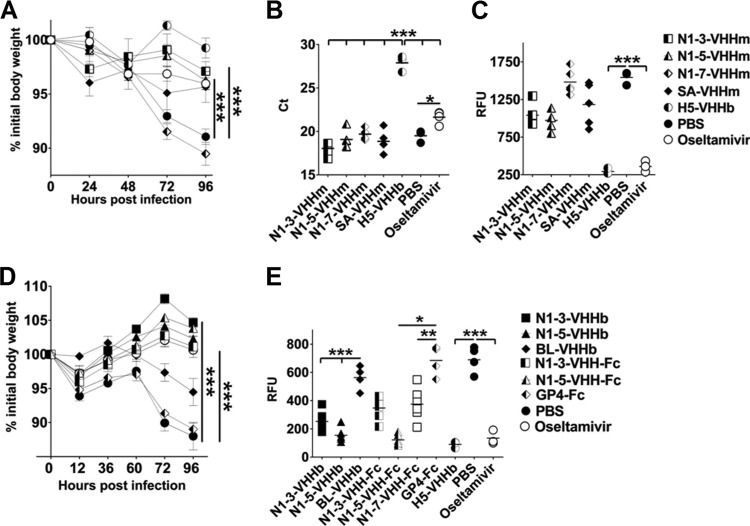
FIG 4.
Prophylactic effect of treatment with inhibitory N1-VHHm, N1-VHHb, and N1-VHH-Fc on H5N1-challenged mice. (A) Groups of 6- to 8-week-old BALB/c mice (n = 5 per group, except oseltamivir and SA-VHHm [n = 4] and H5-VHHb and PBS [n = 3]) were given 100 μg of the N1-VHHm indicated intranasally 4 h before a challenge with 4 LD50s of NIBRG-14ma. Intranasal administration of 30 μg of H5-VHHb and daily oral administration of oseltamivir (45 mg/kg/day, starting 4 h before a challenge) were included as positive controls. Body weight was monitored daily after a challenge and is expressed as a percentage of the initial body weight. (B) Mice were sacrificed on day 4 after a challenge, and lung homogenates were prepared. Viral genomic RNA-specific RT-qPCR was used as a readout of the lung viral load. The CT values of the individual mice are plotted; a horizontal line indicates the mean. (C) AVINA of lung homogenates sampled on day 4 after infection. (D) Bivalent formats of N1-3-VHH and N1-5-VHH administered 4 h before a challenge with 4 LD50s of NIBRG-14ma reduced morbidity. Four hours before a challenge with 4 LD50s of NIBRG-14ma, groups of BALB/c mice (n = 6 per group, except BL-VHHb and PBS [n = 4] and oseltamivir and PBS [n = 3]) were treated intranasally with 60 μg of VHHb (N1-3-VHHb, N1-5-VHHb, or BL-VHHb) or 84 μg of VHH-Fc (N1-3-VHH-Fc, N1-5-VHH-Fc, or GP4-Fc). Mice treated with neutralizing H5-VHHb (30 μg) or oseltamivir (45 mg/kg/day, daily by gavage) were included as positive controls. (E) NA activity in lung homogenates of mice treated with bivalent N1-VHH and challenged with H5N1, as determined by AVINA. In panels A and D, each datum point represents the average body weight of all of the mice alive, and error bars represent standard deviations. Differences between mouse groups were determined by two-way ANOVA. Kaplan-Meier curves were used for survival analysis (*, P < 0.05; **, P < 0.01; ***, P < 0.001). RFU, relative fluorescence units.