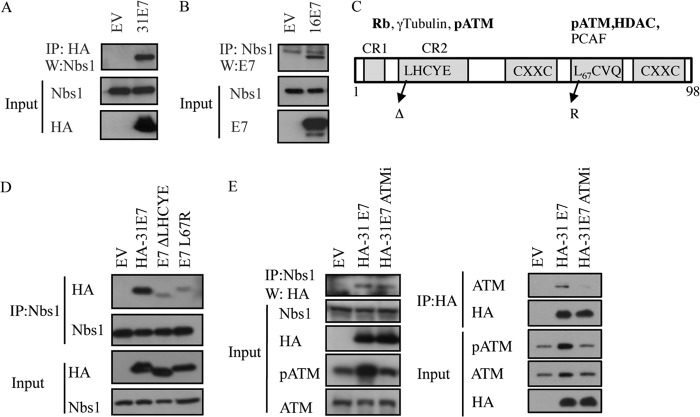
FIG 2.
HPV E7 interacts with Nbs1 independently of ATM. (A) Whole-cell lysates of U20S cells transiently transfected with HA-HPV31 E7 or empty vector (EV) were immunoprecipitated with antibodies to HA or Nbs1, followed by immunoblotting with an antibody to Nbs1 or HA. (B) Whole-cell lysates of U20S cells transiently transfected with TAP-HPV16 E7 or empty vector (EV) were immunoprecipitated using an anti-Nbs1 antibody, followed by immunoblotting with an HPV16 E7 antibody. Inputs were analyzed using antibodies to Nbs1 and HA (A) and Nbs1 and HPV16E7 (B). (C) Structure of E7 and mutations examined in this study. Indicated are cellular targets that have been shown to interact with the Rb (LHCYE) and HDAC (L67) binding domains. Targets in bold have been identified as binding to HPV31 E7 (97). (D) Whole-cell lysates of U20S cells transiently transfected with EV alone, HA-HPV31 E7, HA-HPV31 E7 ΔLHCYE, or HA-HPV31 E7 L67R were immunoprecipitated using an anti-Nbs1 antibody and subsequently immunoblotted using an anti-HA or anti-Nbs1 antibody. Input lysates were analyzed by Western blotting using antibodies to HA and Nbs1. (E) Whole-cell lysates of U20S cells expressing EV alone or HA-HPV31 E7 in the presence or absence of 10 μM ATM inhibitor KU-55933 were immunoprecipitated using an anti-Nbs1 antibody and immunoblotted using an anti-HA antibody (left panel). Input lysates were immunoblotted with antibodies to Nbs1 and HA as well as phosphorylated ATM (Ser1981) and total ATM to demonstrate ATM inhibition. Lysates were also subjected to immunoprecipitation with an antibody to HA, followed by immunoblotting to ATM and HA. Inputs were analyzed using an antibody to HA, as well as phosphorylated and total ATM to demonstrate ATM inhibition. Ab, antibody; IP, immunoprecipitation; W, immunoblotting; ATMi, KU-55933. All results are representative of observations from three or more independent experiments.