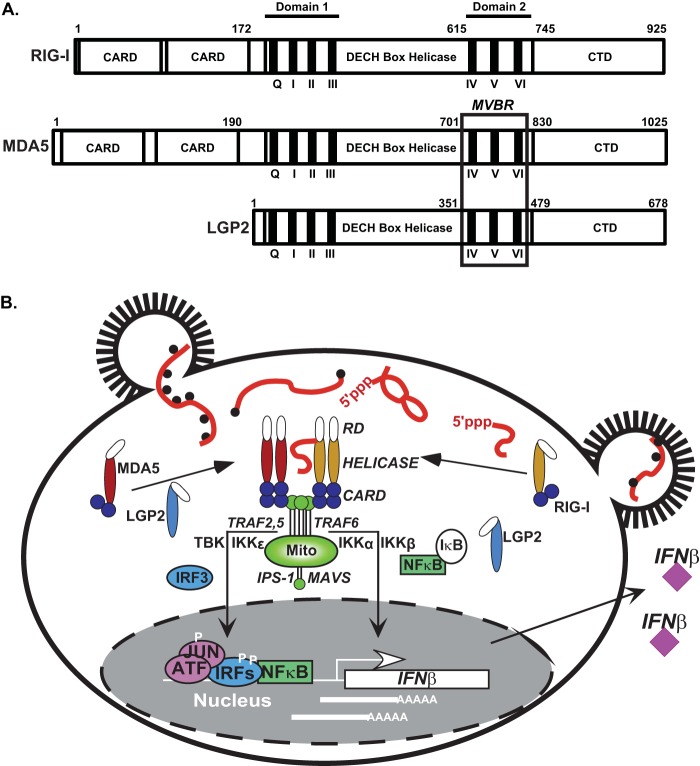
FIG 1.
RLR family proteins in antiviral signaling. (A) Diagram illustrating key features of RIG-I, MDA5, and LGP2 mentioned in the text. The three RLRs are composed of a central DECH box helicase domain containing conserved domain 1 (encompassing helicase motifs Q, I, II, and III) and domain 2 (encompassing helicase motifs IV, V, and VI), both of which are essential to coordinate RNA binding and ATP hydrolysis activities. A C-terminal domain (CTD) is required for RNA terminus recognition and is involved in autoregulation of RIG-I. RIG-I and MDA5 contain tandem caspase activation and recruitment domain (CARD) regions at their N termini, which are essential for downstream signaling activity. The position of the minimal V protein binding region (MVBR), the target site for paramyxovirus V protein antagonism of MDA5 and LGP2, coincides with the boundaries of domain 2. (B) Simplified overview of RLR signal transduction leading to IFN-β gene transcription. Virus infection causes accumulation of cytosolic RNA species with non-self features, including 5′-triphosphates and double-stranded regions (for RIG-I recognition) or long RNAs with structural features (for MDA5 recognition). These RNAs trigger derepression of RIG-I and activate exposure of RLR CARDs to allow interaction with the CARD of the signaling adaptor, IPS-1/MAVS, that is localized on the mitochondria (Mito). Activation of IPS-1/MAVS polymerization stimulates assembly of a signaling scaffold for TRAF2, TRAF5, and TRAF6 and their associated kinases (e.g., TBK, IKKα, IKKβ, and IKKε) that result in phosphorylation-mediated activation of latent IRF3 and NF-κB transcription factors. These factors, along with ATF/JUN, assemble on the IFN-β gene proximal enhancer, resolve nucleosome-mediated repression, and recruit RNA polymerase to induce transcription. Transcribed IFN-β mRNA is translated and subsequently secreted from the cell.