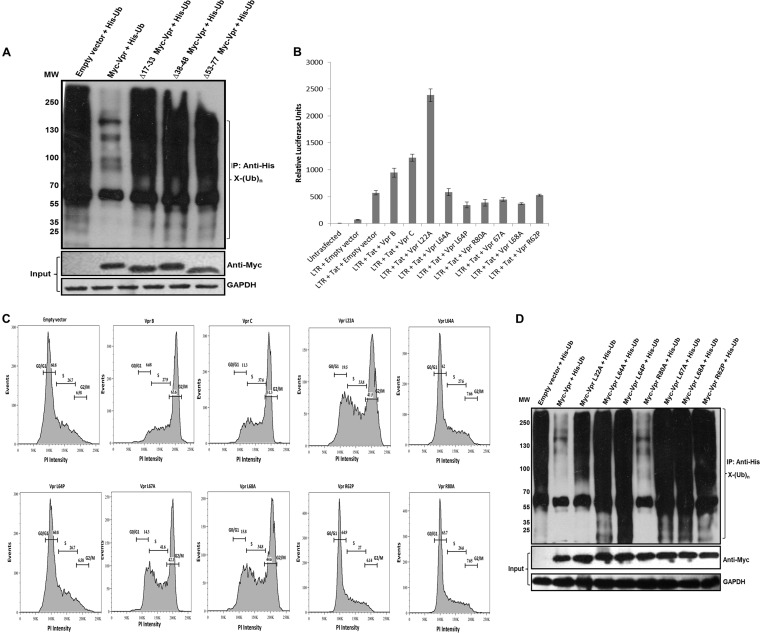
FIG 3.
The three helical regions of Vpr critical for reducing whole-cell ubiquitination. (A) The three helices were independently deleted (Δ17-33, Δ38-48, and Δ53-77 Myc-tagged Vpr deletions). Expression was checked in HEK 293T cells, and each deletion construct was then cotransfected with His-Ub. After 36 h, MG132 treatment was given for 8 h, and ubiquitinated proteins were enriched by using Ni-NTA beads (as described in Materials and Methods). Immunoblotting was done by using an anti-His antibody to probe whole-cell ubiquitination. (B) wt Vpr and point mutants were checked for their abilities to transactivate the LTR by using the LTR-luc construct (as described in Materials and Methods). The results are representative of three independent experiments. (C) wt Vpr and point mutants were checked for their abilities to cause G2/M arrest. HEK 293T cells were collected at 48 h posttransfection and were stained with propidium iodide as described in Materials and Methods. (D) wt Vpr and point mutants were then cotransfected with His-Ub in HEK 293T cells. After 36 h, MG132 treatment was given for 8 h, and ubiquitinated proteins were enriched by using Ni-NTA beads (as described in Materials and Methods). Immunoblotting was done by using an anti-His antibody to probe whole-cell ubiquitination. GAPDH was used as a loading control.