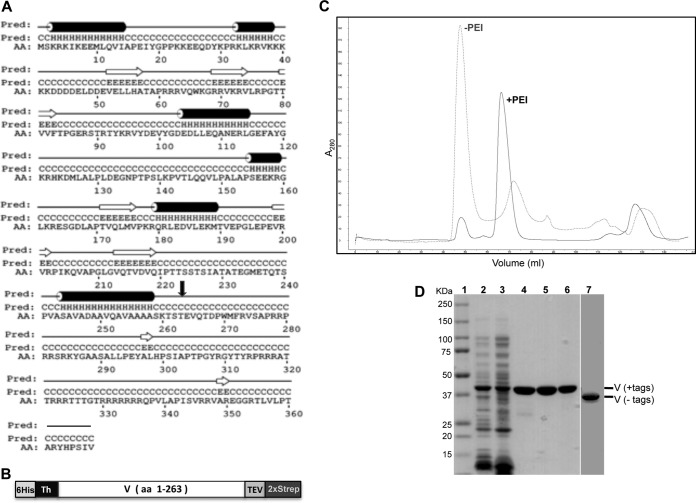
FIG 1.
Expression and purification of recombinant protein V. (A) Secondary structure prediction of protein V with α-helical elements shown as cylinders and β-strands as arrows. The secondary structure prediction was made using PSIPRED V3.3. AA, amino acid sequence; Pred, H-helix, C-coil, and E-strand. The black arrow indicates the residue (T263) where protein V was truncated for expression. (B) Schematic of recombinant protein V used for bacterial expression. The entire construct contains an N-terminal polyhistidine tag (6His), a thrombin (Th) cleavage site, the V coding region (residues 1 to 263), a tobacco etch virus (TEV) cleavage site, and a double Strep-tag (2×Strep) at the C terminus. (C) Chromatogram from SEC showing absorption at 280 nm. The gray elution profile corresponds to protein V purified without PEI, eluting at 48 ml (void volume), while the black profile indicates migration for protein V purified with 0.2% PEI, eluting at 68 ml. (D) SDS-PAGE analyses of protein V at 50 μg (lanes 2 and 3) or 5 μg (lanes 4 to 7) of protein at various stages of purification on 4 to 20% denaturing gels and stained with Simply Blue. Lanes: 1, molecular mass markers; 2, bacterial lysate; 3, supernatant after PEI treatment; 4, elution from Tactin resin; 5, elution from Ni-NTA agarose; 6, protein after SEC; 7, protein treated with thrombin and TEV to remove the tags after SEC.